Targeting a xenobiotic transporter to ameliorate vincristine-induced sensory neuropathy
- PMID: 37347545
- PMCID: PMC10443802
- DOI: 10.1172/jci.insight.164646
Targeting a xenobiotic transporter to ameliorate vincristine-induced sensory neuropathy
Abstract
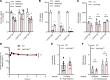
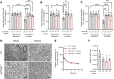
Vincristine is a widely used chemotherapeutic drug for the treatment of multiple malignant diseases that causes a dose-limiting peripheral neurotoxicity. There is no clinically effective preventative treatment for vincristine-induced sensory peripheral neurotoxicity (VIPN), and mechanistic details of this side effect remain poorly understood. We hypothesized that VIPN is dependent on transporter-mediated vincristine accumulation in dorsal root ganglion neurons. Using a xenobiotic transporter screen, we identified OATP1B3 as a neuronal transporter regulating the uptake of vincristine. In addition, genetic or pharmacological inhibition of the murine orthologue transporter OATP1B2 protected mice from various hallmarks of VIPN - including mechanical allodynia, thermal hyperalgesia, and changes in digital maximal action potential amplitudes and neuronal morphology - without negatively affecting plasma levels or antitumor effects of vincristine. Finally, we identified α-tocopherol from an untargeted metabolomics analysis as a circulating endogenous biomarker of neuronal OATP1B2 function, and it could serve as a companion diagnostic to guide dose selection of OATP1B-type transport modulators given in combination with vincristine to prevent VIPN. Collectively, our findings shed light on the fundamental basis of VIPN and provide a rationale for the clinical development of transporter inhibitors to prevent this debilitating side effect.
Keywords: Oncology; Pharmacology; Toxins/drugs/xenobiotics; Transport.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

