CES1 Releases Oxylipins from Oxidized Triacylglycerol (oxTAG) and Regulates Macrophage oxTAG/TAG Accumulation and PGE2/IL-1β Production
- PMID: 37348046
- PMCID: PMC11131412
- DOI: 10.1021/acschembio.3c00194
CES1 Releases Oxylipins from Oxidized Triacylglycerol (oxTAG) and Regulates Macrophage oxTAG/TAG Accumulation and PGE2/IL-1β Production
Abstract
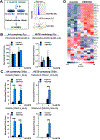
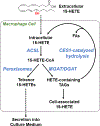
Triacylglycerols (TAGs) are storage forms of fat, primarily found in cytoplasmic lipid droplets in cells. TAGs are broken down to their component free fatty acids by lipolytic enzymes when fuel reserves are required. However, polyunsaturated fatty acid (PUFA)-containing TAGs are susceptible to nonenzymatic oxidation reactions, leading to the formation of oxylipins that are esterified to the glycerol backbone (termed oxTAGs). Human carboxylesterase 1 (CES1) is a member of the serine hydrolase superfamily and defined by its ability to catalyze the hydrolysis of carboxyl ester bonds in both toxicants and lipids. CES1 is a bona fide TAG hydrolase, but it is unclear which specific fatty acids are preferentially released during lipolysis. To better understand the biochemical function of CES1 in immune cells, such as macrophages, its substrate selectivity when it encounters oxidized PUFAs in TAG lipid droplets requires study. We sought to identify those esterified oxidized fatty acids liberated from oxTAGs by CES1 because their release can activate signaling pathways that enforce the development of lipid-driven inflammation. Gaining this knowledge will help fill data gaps that exist between CES1 and the lipid-sensing nuclear receptors, PPARγ and LXRα, which are important drivers of lipid metabolism and inflammation in macrophages. Oxidized forms of triarachidonoylglycerol (oxTAG20:4) or trilinoleoylglycerol (oxTAG18:2), which contain physiologically relevant levels of oxidized PUFAs (<5 mol %), were incubated with recombinant CES1 to release oxylipins and nonoxidized arachidonic acid (AA) or linoleic acid (LA). CES1 hydrolyzed each oxTAG, yielding regioisomers of hydroxyeicosatetraenoic acids (5-, 11-, 12-, and 15-HETE) and hydroxyoctadecadienoic acids (9- and 13-HODE). Furthermore, human THP-1 macrophages with deficient CES1 levels exhibited a differential response to extracellular stimuli (oxTAGs, lipopolysaccharide, and 15-HETE) as compared to those with normal CES1 levels, including enhanced oxTAG/TAG lipid accumulation and altered cytokine and prostaglandin E2 profiles. This study suggests that CES1 can metabolize oxTAG lipids to release oxylipins and PUFAs, and it further specifies the substrate selectivity of CES1 in the metabolism of bioactive lipid mediators. We suggest that the accumulation of oxTAGs/TAGs within lipid droplets that arise due to CES1 deficiency enforces an inflammatory phenotype in macrophages.
Figures




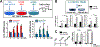



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

