Randomized phase II adjuvant trial to compare two treatment durations of icotinib (2 years versus 1 year) for stage II-IIIA EGFR-positive lung adenocarcinoma patients (ICOMPARE study)
- PMID: 37348348
- PMCID: PMC10515286
- DOI: 10.1016/j.esmoop.2023.101565
Randomized phase II adjuvant trial to compare two treatment durations of icotinib (2 years versus 1 year) for stage II-IIIA EGFR-positive lung adenocarcinoma patients (ICOMPARE study)
Abstract
Background: Despite the prolonged median disease-free survival (DFS) by adjuvant targeted therapy in non-small-cell lung cancer patients with epidermal growth factor receptor (EGFR) mutations, the relationship between the treatment duration and the survival benefits in patients remains unknown.
Patients and methods: In this multicenter, randomized, open-label, phase II trial, eligible patients aged 18-75 years with EGFR-mutant, stage II-IIIA lung adenocarcinoma and who had not received adjuvant chemotherapy after complete tumor resection were enrolled from eight centers in China. Patients were randomly assigned (1 : 1) to receive either 1-year or 2-year icotinib (125 mg thrice daily). The primary endpoint was DFS assessed by investigator. The secondary endpoints were overall survival (OS) and safety. This study was registered at ClinicalTrials.gov (NCT01929200).
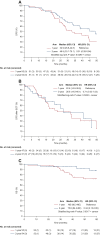
Results: Between September 2013 and October 2018, 109 patients were enrolled (1-year group, n = 55; 2-year group, n = 54). Median DFS was 48.9 months [95% confidence interval (CI) 33.1-70.1 months] in the 2-year group and 32.9 months (95% CI 26.6-44.8 months) in the 1-year group [hazard ratio (HR) 0.51; 95% CI 0.28-0.94; P = 0.0290]. Median OS for patients was 75.8 months [95% CI 64.4 months-not evaluable (NE)] in the 2-year group and NE (95% CI 66.3 months-NE) in the 1-year group (HR 0.34; 95% CI 0.13-0.95; P = 0.0317). Treatment-related adverse events (TRAEs) were observed in 41 of 55 (75%) patients in the 1-year group and in 36 of 54 (67%) patients in the 2-year group. Grade 3-4 TRAEs occurred in 4 of 55 (7%) patients in the 1-year group and in 3 of 54 (6%) patients in the 2-year group. No treatment-related deaths or interstitial lung disease was reported.
Conclusions: Two-year adjuvant icotinib was shown to significantly improve DFS and provide an OS benefit in EGFR-mutant, stage II-IIIA lung adenocarcinoma patients compared with 1-year treatment in this exploratory phase II study.
Keywords: EGFR mutations; adjuvant targeted therapy; disease-free survival; icotinib; non-small-cell lung cancer.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Xu S.T., Xi J.J., Zhong W.Z., et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: a post hoc analysis of the ADJUVANT trial (CTONG 1104) J Thorac Oncol. 2019;14:503–512. - PubMed
-
- Goldstraw P., Chansky K., Crowley J., et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. - PubMed
-
- Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

