Input density tunes Kenyon cell sensory responses in the Drosophila mushroom body
- PMID: 37348501
- PMCID: PMC10529417
- DOI: 10.1016/j.cub.2023.05.064
Input density tunes Kenyon cell sensory responses in the Drosophila mushroom body
Abstract
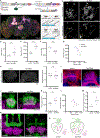
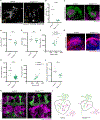
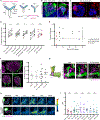
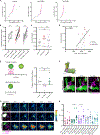
The ability to discriminate sensory stimuli with overlapping features is thought to arise in brain structures called expansion layers, where neurons carrying information about sensory features make combinatorial connections onto a much larger set of cells. For 50 years, expansion coding has been a prime topic of theoretical neuroscience, which seeks to explain how quantitative parameters of the expansion circuit influence sensory sensitivity, discrimination, and generalization. Here, we investigate the developmental events that produce the quantitative parameters of the arthropod expansion layer, called the mushroom body. Using Drosophila melanogaster as a model, we employ genetic and chemical tools to engineer changes to circuit development. These allow us to produce living animals with hypothesis-driven variations on natural expansion layer wiring parameters. We then test the functional and behavioral consequences. By altering the number of expansion layer neurons (Kenyon cells) and their dendritic complexity, we find that input density, but not cell number, tunes neuronal odor selectivity. Simple odor discrimination behavior is maintained when the Kenyon cell number is reduced and augmented by Kenyon cell number expansion. Animals with increased input density to each Kenyon cell show increased overlap in Kenyon cell odor responses and become worse at odor discrimination tasks.
Keywords: Drosophila melangaster; Marr-Albus theory; brain engineering; development; expansion layer; mushroom body; neural network; olfaction; pattern separation.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Wong AM, Wang JW, and Axel R (2002). Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 109, 229–241. - PubMed
-
- Marin EC, Jefferis GSXE, Komiyama T, Zhu H, and Luo L (2002). Representation of the glomerular olfactory map in the Drosophila brain. Cell 109, 243–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

