PD-L1/PD-1 checkpoint pathway regulates hippocampal neuronal excitability and learning and memory behavior
- PMID: 37348508
- PMCID: PMC10529885
- DOI: 10.1016/j.neuron.2023.05.022
PD-L1/PD-1 checkpoint pathway regulates hippocampal neuronal excitability and learning and memory behavior
Abstract
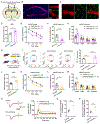
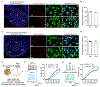
Programmed death protein 1 (PD-1) and its ligand PD-L1 constitute an immune checkpoint pathway. We report that neuronal PD-1 signaling regulates learning/memory in health and disease. Mice lacking PD-1 (encoded by Pdcd1) exhibit enhanced long-term potentiation (LTP) and memory. Intraventricular administration of anti-mouse PD-1 monoclonal antibody (RMP1-14) potentiated learning and memory. Selective deletion of PD-1 in excitatory neurons (but not microglia) also enhances LTP and memory. Traumatic brain injury (TBI) impairs learning and memory, which is rescued by Pdcd1 deletion or intraventricular PD-1 blockade. Conversely, re-expression of Pdcd1 in PD-1-deficient hippocampal neurons suppresses memory and LTP. Exogenous PD-L1 suppresses learning/memory in mice and the excitability of mouse and NHP hippocampal neurons through PD-1. Notably, neuronal activation suppresses PD-L1 secretion, and PD-L1/PD-1 signaling is distinctly regulated by learning and TBI. Thus, conditions that reduce PD-L1 levels or PD-1 signaling could promote memory in both physiological and pathological conditions.
Keywords: hippocampal neurons; immunotherapy; long-term potentiation; mice; microglia; nonhuman primate; programmed cell death ligand 1; programmed cell death protein 1; traumatic brain injury.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, and Schwartz M (2016). PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med 22, 135–137. 10.1038/nm.4022. - DOI - PubMed
-
- Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, Cahalon L, Kertser A, Baruch K, Amit I, et al. (2019). PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun 10, 465. 10.1038/s41467-019-08352-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

