Short intravenous amphotericin B followed by oral posaconazole using a simple, stratified treatment approach for diabetes or COVID-19-associated rhino-orbito-cerebral mucormycosis: a prospective cohort study
- PMID: 37348653
- PMCID: PMC10281032
- DOI: 10.1016/j.cmi.2023.06.017
Short intravenous amphotericin B followed by oral posaconazole using a simple, stratified treatment approach for diabetes or COVID-19-associated rhino-orbito-cerebral mucormycosis: a prospective cohort study
Abstract
Objectives: To evaluate the efficacy and safety of short-course intravenous amphotericin B followed by sustained release posaconazole tablets for diabetes or COVID-19-associated rhino-orbito-cerebral mucormycosis.
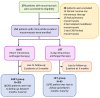
Methods: This prospective, pragmatic study included adults with diabetes or COVID-19-associated rhino-orbito-cerebral mucormycosis. Patients received short (7-14 days) or long (15-28 days) intravenous antifungal therapy (short intravenous antifungal treatment [SHIFT] or long intravenous antifungal treatment [LIFT], respectively) depending on the presence or absence of brain involvement. All patients received step-down posaconazole tablets, debridement, and glycemic control. The primary outcome was the treatment success at week 14, which was determined by assessing survival and the absence of disease progression through clinical evaluation and nasal endoscopy. Log-binomial regression analysis (risk ratio and 95% CI) was performed to assess factors associated with the primary outcome.
Results: Intravenous therapy was administered to 251 participants: SHIFT, 205 (median duration, 13 days); LIFT, 46 (median duration, 22 days). Treatment success at 3 months was 88% (217/248; 95% CI, 83-91%): SHIFT group, 93% (189/203; 89-96%); LIFT group, 62% (28/45; 47-76%). All-cause mortality was 12% (30/251): SHIFT group, 6% (13/205); LIFT group, 37% (17/46). Age (aRR [95% CI]: 1.02 [1.00-1.05]; p 0.027), diabetic ketoacidosis at presentation (2.32 [1.20-4.46]; p 0·012), glycated haemoglobin A1c (1.19 [1.03-1.39]; p 0.019), stroke (3.93 [1.94-7.95]; p 0·0001), and brain involvement (5.67 [3.05-10.54]; p < 0.0001) were independently associated with unsuccessful outcomes.
Discussion: Short intravenous amphotericin B with step-down posaconazole tablets should be further studied as primary treatment option for diabetes or COVID-19-associated mucormycosis in randomized controlled trials.
Keywords: Diabetes-associated mucormycosis; Lipid emulsion amphotericin B; Oral posaconazole; Rhino-orbito-cerebral; Short intravenous amphotericin B.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
References
-
- Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical