Onco-condensates: formation, multi-component organization, and biological functions
- PMID: 37349246
- PMCID: PMC10524369
- DOI: 10.1016/j.trecan.2023.05.006
Onco-condensates: formation, multi-component organization, and biological functions
Abstract
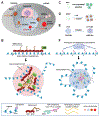
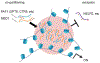
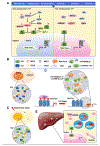
Numerous cellular processes occur in the context of condensates, a type of large, membrane-less biomolecular assembly generated through phase separation. These condensates function as a hub of diversified cellular events by concentrating the required components. Cancer frequently coopts biomolecular condensation mechanisms to promote survival and/or proliferation. Onco-condensates, which refer to those that have causal roles or are critically involved in tumorigenicity, operate to abnormally elevate biological output of a proliferative process, or to suppress a tumor-suppressive pathway, thereby promoting oncogenesis. Here, we summarize advances regarding how multi-component onco-condensates are established and organized to promote oncogenesis, with those related to chromatin and transcription deregulation used as showcases. A better understanding should enable development of new means of targeting onco-condensates as potential therapeutics.
Keywords: biomolecular condensation; cancer; disease; intrinsically disordered region (IDR); onco-condensate; phase separation; small molecule; therapeutic.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no interests to declare.
Figures
Similar articles
-
Biomolecular Condensates and Cancer.Cancer Cell. 2021 Feb 8;39(2):174-192. doi: 10.1016/j.ccell.2020.12.003. Epub 2021 Jan 7. Cancer Cell. 2021. PMID: 33417833 Free PMC article. Review.
-
Liquid‒liquid phase separation: roles and implications in future cancer treatment.Int J Biol Sci. 2023 Aug 6;19(13):4139-4156. doi: 10.7150/ijbs.81521. eCollection 2023. Int J Biol Sci. 2023. PMID: 37705755 Free PMC article. Review.
-
Regulation of Polyhomeotic Condensates by Intrinsically Disordered Sequences That Affect Chromatin Binding.Epigenomes. 2022 Nov 3;6(4):40. doi: 10.3390/epigenomes6040040. Epigenomes. 2022. PMID: 36412795 Free PMC article.
-
Decoding the genomic landscape of chromatin-associated biomolecular condensates.Nat Commun. 2024 Aug 13;15(1):6952. doi: 10.1038/s41467-024-51426-2. Nat Commun. 2024. PMID: 39138204 Free PMC article.
-
Defining the condensate landscape of fusion oncoproteins.Nat Commun. 2023 Sep 28;14(1):6008. doi: 10.1038/s41467-023-41655-2. Nat Commun. 2023. PMID: 37770423 Free PMC article.
Cited by
-
The sotos syndrome gene Nsd1 safeguards developmental gene enhancers poised for transcription by maintaining the precise deposition of histone methylation.J Biol Chem. 2025 May;301(5):108423. doi: 10.1016/j.jbc.2025.108423. Epub 2025 Mar 19. J Biol Chem. 2025. PMID: 40118455 Free PMC article.
-
Global research hotspots, development trends and prospect discoveries of phase separation in cancer: a decade-long informatics investigation.Biomark Res. 2024 Apr 16;12(1):39. doi: 10.1186/s40364-024-00587-9. Biomark Res. 2024. PMID: 38627840 Free PMC article.
-
A Minireview on BET Inhibitors: Beyond Bromodomain Targeting.Biomedicines. 2025 Mar 1;13(3):594. doi: 10.3390/biomedicines13030594. Biomedicines. 2025. PMID: 40149571 Free PMC article. Review.
-
Phase Separation in Chromatin Organization and Human Diseases.Int J Mol Sci. 2025 May 28;26(11):5156. doi: 10.3390/ijms26115156. Int J Mol Sci. 2025. PMID: 40507965 Free PMC article. Review.
-
Pharmacologic degradation of WDR5 suppresses oncogenic activities of SS18::SSX and provides a therapeutic of synovial sarcoma.Sci Adv. 2025 Apr 25;11(17):eads7876. doi: 10.1126/sciadv.ads7876. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267190 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

