LC-MS/MS based characterisation and differential expression of proteins in Himalayan snow trout, Schizothorax labiatus using LFQ technique
- PMID: 37349327
- PMCID: PMC10287682
- DOI: 10.1038/s41598-023-35646-y
LC-MS/MS based characterisation and differential expression of proteins in Himalayan snow trout, Schizothorax labiatus using LFQ technique
Erratum in
-
Author Correction: LC-MS/MS based characterisation and differential expression of proteins in Himalayan snow trout, Schizothorax labiatus using LFQ technique.Sci Rep. 2023 Jul 18;13(1):11591. doi: 10.1038/s41598-023-38786-3. Sci Rep. 2023. PMID: 37464075 Free PMC article. No abstract available.
Abstract
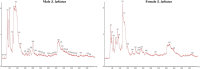
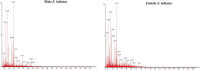
Molecular characterization of fish muscle proteins are nowadays considered as a key component to understand the role of specific proteins involved in various physiological and metabolic processes including their up and down regulation in the organisms. Coldwater fish specimens including snow trouts hold different types of proteins which help them to survive in highly diversified temperatures fluctuating from 0 to 20 °C. So, in current study, the liquid chromatography mass spectrometry using label free quantification technique has been used to investigate the muscle proteome profile of Schizothorax labiatus. For proteomic study, two weight groups of S. labiatus were taken from river Sindh. The proteomic analysis of group 1 revealed that a total of 235 proteins in male and 238 in female fish were recorded. However, when male and female S. labiatus were compared with each other on the basis of spectral count and abundance of peptides by ProteinLynx Global Server software, a total of 14 down-regulated and 22 up-regulated proteins were noted in this group. The highly down-regulated ones included homeodomain protein HoxA2b, retinol-binding protein 4, MHC class II beta chain and proopiomelanocortin while as the highly expressed up-regulated proteins comprised of gonadotropin I beta subunit, NADH dehydrogenase subunit 4, manganese superoxide dismutase, recombinase-activating protein 2, glycosyltransferase, chymotrypsin and cytochrome b. On the other hand, the proteomic characterisation of group 2 of S. labiatus revealed that a total of 227 proteins in male and 194 in female fish were recorded. When male and female S. labiatus were compared with each other by label free quantification, a total of 20 down-regulated and 18 up-regulated proteins were recorded. The down-regulated protein expression of group 2 comprised hepatic lipase, allograft inflammatory factor-1, NADH dehydrogenase subunit 4 and myostatin 1 while the highly expressed up-regulated proteins included glycogen synthase kinase-3 beta variant 2, glycogen synthase kinase-3 beta variant 5, cholecystokinin, glycogen synthase kinase-3 beta variant 3 and cytochrome b. Significant (P < 0.05) difference in the expression of down-regulated and up-regulated proteins was also noted between the two sexes of S. labiatus in each group. According to MS analysis, the proteins primarily concerned with the growth, skeletal muscle development and metabolism were down-regulated in river Sindh, which indicates that growth of fish during the season of collection i.e., winter was slow owing to less food availability, gonad development and low metabolic activity. While, the proteins related to immune response of fish were also noted to be down-regulated thereby signifying that the ecosystem has less pollution loads, microbial, pathogenic and anthropogenic activities. It was also found that the proteins involved in glycogen metabolism, reproductive and metabolic processes, particularly lipid metabolism were up-regulated in S. labiatus. The significant expression of these proteins may be connected to pre-spawning, gonad development and use of stored food as source of energy. The information generated in this study can be applied to future research aimed at enhancing food traceability, food safety, risk management and authenticity analysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Jagadeesh DS, Kannegundla U, Reddy RK. Application of proteomic tools in food quality and safety. Adv. Anim. Vet. Sci. 2017;5:213–225. doi: 10.17582/journal.aavs/2017/5.5.213.225. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

