Electron cooling in graphene enhanced by plasmon-hydron resonance
- PMID: 37349505
- PMCID: PMC10427419
- DOI: 10.1038/s41565-023-01421-3
Electron cooling in graphene enhanced by plasmon-hydron resonance
Abstract
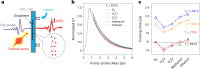
Evidence is accumulating for the crucial role of a solid's free electrons in the dynamics of solid-liquid interfaces. Liquids induce electronic polarization and drive electric currents as they flow; electronic excitations, in turn, participate in hydrodynamic friction. Yet, the underlying solid-liquid interactions have been lacking a direct experimental probe. Here we study the energy transfer across liquid-graphene interfaces using ultrafast spectroscopy. The graphene electrons are heated up quasi-instantaneously by a visible excitation pulse, and the time evolution of the electronic temperature is then monitored with a terahertz pulse. We observe that water accelerates the cooling of the graphene electrons, whereas other polar liquids leave the cooling dynamics largely unaffected. A quantum theory of solid-liquid heat transfer accounts for the water-specific cooling enhancement through a resonance between the graphene surface plasmon mode and the so-called hydrons-water charge fluctuations-particularly the water libration modes, which allows for efficient energy transfer. Our results provide direct experimental evidence of a solid-liquid interaction mediated by collective modes and support the theoretically proposed mechanism for quantum friction. They further reveal a particularly large thermal boundary conductance for the water-graphene interface and suggest strategies for enhancing the thermal conductivity in graphene-based nanostructures.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Romagnoli M, et al. Graphene-based integrated photonics for next-generation datacom and telecom. Nat. Rev. Mater. 2018;3:392–414. doi: 10.1038/s41578-018-0040-9. - DOI
Grants and funding
- 873028 HYDROTRONICS/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- RPG-2019-363/Leverhulme Trust
- 804349/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- MaxWater/Max-Planck-Gesellschaft (Max Planck Society)
LinkOut - more resources
Full Text Sources

