Single-cell m6A mapping in vivo using picoMeRIP-seq
- PMID: 37349523
- PMCID: PMC10739642
- DOI: 10.1038/s41587-023-01831-7
Single-cell m6A mapping in vivo using picoMeRIP-seq
Abstract
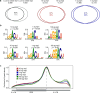
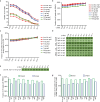
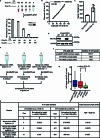
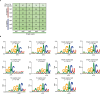
Current N6-methyladenosine (m6A) mapping methods need large amounts of RNA or are limited to cultured cells. Through optimized sample recovery and signal-to-noise ratio, we developed picogram-scale m6A RNA immunoprecipitation and sequencing (picoMeRIP-seq) for studying m6A in vivo in single cells and scarce cell types using standard laboratory equipment. We benchmark m6A mapping on titrations of poly(A) RNA and embryonic stem cells and in single zebrafish zygotes, mouse oocytes and embryos.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

