Effects of tolvaptan discontinuation in patients with autosomal dominant polycystic kidney disease: a post hoc pooled analysis
- PMID: 37349694
- PMCID: PMC10286436
- DOI: 10.1186/s12882-023-03247-6
Effects of tolvaptan discontinuation in patients with autosomal dominant polycystic kidney disease: a post hoc pooled analysis
Abstract
Background: Tolvaptan slows kidney function decline in patients with autosomal dominant polycystic kidney disease (ADPKD) who are at risk of rapid progression. Given that treatment requires commitment to long-term use, we evaluated the effects of tolvaptan discontinuation on the trajectory of ADPKD progression.
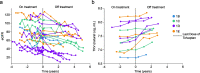
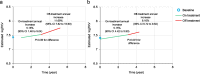
Methods: This was a post hoc analysis of pooled data from two clinical trials of tolvaptan (TEMPO 2:4 [NCT00413777] and TEMPO 3:4 [NCT00428948]), an extension trial (TEMPO 4:4 [NCT01214421]), and an observational study (OVERTURE [NCT01430494]) that enrolled patients from the other trials. Individual subject data were linked longitudinally across trials to construct analysis cohorts of subjects with a tolvaptan treatment duration > 180 days followed by an off-treatment observation period of > 180 days. For inclusion in Cohort 1, subjects were required have ≥ 2 outcome assessments during the tolvaptan treatment period and ≥ 2 assessments during the follow-up period. For Cohort 2, subjects were required to have ≥ 1 assessment during the tolvaptan treatment period and ≥ 1 assessment during the follow-up period. Outcomes were rates of change in estimated glomerular filtration rate (eGFR) and total kidney volume (TKV). Piecewise-mixed models compared changes in eGFR or TKV in the on-treatment and post-treatment periods.
Results: In the Cohort 1 eGFR population (n = 20), the annual rate of eGFR change (in mL/min/1.73 m2) was -3.18 on treatment and -4.33 post-treatment, a difference that was not significant (P = 0.16), whereas in Cohort 2 (n = 82), the difference between on treatment (-1.89) and post-treatment (-4.94) was significant (P < 0.001). In the Cohort 1 TKV population (n = 11), TKV increased annually by 5.18% on treatment and 11.69% post-treatment (P = 0.06). In Cohort 2 (n = 88), the annual TKV growth rates were 5.15% on treatment and 8.16% post-treatment (P = 0.001).
Conclusions: Although limited by small sample sizes, these analyses showed directionally consistent acceleration in measures of ADPKD progression following the discontinuation of tolvaptan.
Keywords: Autosomal dominant polycystic kidney disease (ADPKD); Clinical trial; Glomerular filtration rate; Kidney volume; Tolvaptan; Treatment.
© 2023. The Author(s).
Conflict of interest statement
ML is a member of a physician advisory board for Otsuka Pharmaceutical.
XZ and ED are employees of RTI Health Solutions (Research Triangle Park, NC), which was contracted by Otsuka to perform the analyses.
SN, DO, and AWF are employees of Otsuka Pharmaceutical Development & Commercialization, Inc (Princeton, NJ, USA).
HBK: employee of Otsuka 2001–2019; consultant for Otsuka 2019–2021.
Figures


References
-
- Müller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O, et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2022;37(5):825–839. doi: 10.1093/ndt/gfab312. - DOI - PMC - PubMed
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Dandurand A, et al. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2018;33(3):477–489. doi: 10.1093/ndt/gfx043. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

