Clinical efficacy of denosumab, teriparatide, and oral bisphosphonates in the prevention of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis
- PMID: 37349750
- PMCID: PMC10286508
- DOI: 10.1186/s13018-023-03920-4
Clinical efficacy of denosumab, teriparatide, and oral bisphosphonates in the prevention of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis
Abstract
Background: Continuous use of glucocorticoids (GCs) has become the primary cause of secondary osteoporosis. Bisphosphonate drugs were given priority over denosumab and teriparatide in the 2017 American College of Rheumatology (ACR) guidelines but have a series of shortcomings. This study aims to explore the efficacy and safety of teriparatide and denosumab compared with those of oral bisphosphonate drugs.
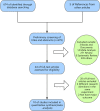
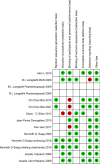
Methods: We systematically searched studies included in the PubMed, Web of Science, Embase, and Cochrane library databases and included randomized controlled trials that compared denosumab or teriparatide with oral bisphosphonates. Risk estimates were pooled using both fixed and random effects models.
Results: We included 10 studies involving 2923 patients who received GCs for meta-analysis, including two drug base analyses and four sensitivity analyses. Teriparatide and denosumab were superior to bisphosphonates in increasing the bone mineral density (BMD) of the lumbar vertebrae [teriparatide: mean difference [MD] 3.98%, 95% confidence interval [CI] 3.61-4.175%, P = 0.00001; denosumab: MD 2.07%, 95% CI 0.97-3.17%, P = 0.0002]. Teriparatide was superior to bisphosphonates in preventing vertebral fractures and increasing hip BMD [MD 2.39%, 95% CI 1.47-3.32, P < 0.00001]. There was no statistically significant difference between serious adverse events, adverse events, and nonvertebral fracture prevention drugs.
Conclusions: Teriparatide and denosumab exhibited similar or even superior characteristics to bisphosphonates in our study, and we believe that they have the potential to become first-line GC-induced osteoporosis treatments, especially for patients who have previously received other anti-osteoporotic drugs with poor efficacy.
Keywords: Bisphosphonates; Denosumab; Glucocorticoid; Osteoporosis; Teriparatide.
© 2023. The Author(s).
Conflict of interest statement
The authors declared that there were no competing interests in the study.
Figures






Similar articles
-
Efficacy and safety of denosumab and teriparatide versus oral bisphosphonates to treat postmenopausal osteoporosis: a systematic review and meta-analysis.Front Endocrinol (Lausanne). 2024 Sep 2;15:1431676. doi: 10.3389/fendo.2024.1431676. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39286276 Free PMC article.
-
Efficacy and safety of teriparatide vs. bisphosphonates and denosumab vs. bisphosphonates in osteoporosis not previously treated with bisphosphonates: a systematic review and meta-analysis of randomized controlled trials.Arch Osteoporos. 2024 Sep 23;19(1):89. doi: 10.1007/s11657-024-01447-7. Arch Osteoporos. 2024. PMID: 39312040 Free PMC article.
-
Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation.Health Technol Assess. 2020 Jun;24(29):1-314. doi: 10.3310/hta24290. Health Technol Assess. 2020. PMID: 32588816 Free PMC article.
-
Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis.BMC Musculoskelet Disord. 2022 Nov 29;23(1):1027. doi: 10.1186/s12891-022-05997-0. BMC Musculoskelet Disord. 2022. PMID: 36447169 Free PMC article.
-
Four-Year Teriparatide Followed by Denosumab vs. Continuous Denosumab in Glucocorticoid-Induced Osteoporosis Patients With Prior Bisphosphonate Treatment.Front Endocrinol (Lausanne). 2021 Sep 27;12:753185. doi: 10.3389/fendo.2021.753185. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34646240 Free PMC article. Clinical Trial.
Cited by
-
Rodents as an animal model for studying tooth extraction-related medication-related osteonecrosis of the jaw: assessment of outcomes.Arch Oral Biol. 2024 Mar;159:105875. doi: 10.1016/j.archoralbio.2023.105875. Epub 2023 Dec 26. Arch Oral Biol. 2024. PMID: 38160519 Free PMC article. Review.
-
The osteocytic actions of glucocorticoids on bone mass, mechanical properties, or perilacunar remodeling outcomes are not rescued by PTH(1-34).Front Endocrinol (Lausanne). 2024 Jul 18;15:1342938. doi: 10.3389/fendo.2024.1342938. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39092287 Free PMC article.
-
Extrapulmonary Comorbidities Associated with Chronic Obstructive Pulmonary Disease: A Review.Int J Chron Obstruct Pulmon Dis. 2024 Feb 29;19:567-578. doi: 10.2147/COPD.S447739. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38476124 Free PMC article. Review.
-
Efficacy and safety of denosumab and teriparatide versus oral bisphosphonates to treat postmenopausal osteoporosis: a systematic review and meta-analysis.Front Endocrinol (Lausanne). 2024 Sep 2;15:1431676. doi: 10.3389/fendo.2024.1431676. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39286276 Free PMC article.
-
Denosumab, teriparatide and bisphosphonates for glucocorticoid-induced osteoporosis: a Bayesian network meta-analysis.Front Pharmacol. 2024 Jan 19;15:1336075. doi: 10.3389/fphar.2024.1336075. eCollection 2024. Front Pharmacol. 2024. PMID: 38313307 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

