Effectiveness of laparoscopic removal of isolated superficial peritoneal endometriosis for the management of chronic pelvic pain in women (ESPriT2): protocol for a multi-centre randomised controlled trial
- PMID: 37349849
- PMCID: PMC10286505
- DOI: 10.1186/s13063-023-07386-x
Effectiveness of laparoscopic removal of isolated superficial peritoneal endometriosis for the management of chronic pelvic pain in women (ESPriT2): protocol for a multi-centre randomised controlled trial
Abstract
Background: Endometriosis affects 190 million women and those assigned female at birth worldwide. For some, it is associated with debilitating chronic pelvic pain. Diagnosis of endometriosis is often achieved through diagnostic laparoscopy. However, when isolated superficial peritoneal endometriosis (SPE), the most common endometriosis subtype, is identified during laparoscopy, limited evidence exists to support the common decision to surgically remove it via excision or ablation. Improved understanding of the impact of surgical removal of isolated SPE for the management of chronic pelvic pain in women is required. Here, we describe our protocol for a multi-centre trial to determine the effectiveness of surgical removal of isolated SPE for the management of endometriosis-associated pain.
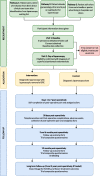
Methods: We plan to undertake a multi-centre participant-blind parallel-group randomised controlled clinical and cost-effectiveness trial with internal pilot. We plan to randomise 400 participants from up to 70 National Health Service Hospitals in the UK. Participants with chronic pelvic pain awaiting diagnostic laparoscopy for suspected endometriosis will be consented by the clinical research team. If isolated SPE is identified at laparoscopy, and deep or ovarian endometriosis is not seen, participants will be randomised intraoperatively (1:1) to surgical removal (by excision or ablation or both, according to surgeons' preference) versus diagnostic laparoscopy alone. Randomisation with block-stratification will be used. Participants will be given a diagnosis but will not be informed of the procedure they received until 12 months post-randomisation, unless required. Post-operative medical treatment will be according to participants' preference. Participants will be asked to complete validated pain and quality of life questionnaires at 3, 6 and 12 months after randomisation. Our primary outcome is the pain domain of the Endometriosis Health Profile-30 (EHP-30), via a between randomised group comparison of adjusted means at 12 months. Assuming a standard deviation of 22 points around the pain score, 90% power, 5% significance and 20% missing data, 400 participants are required to be randomised to detect an 8-point pain score difference.
Discussion: This trial aims to provide high quality evidence of the clinical and cost-effectiveness of surgical removal of isolated SPE.
Trial registration: ISRCTN registry ISRCTN27244948. Registered 6 April 2021.
Keywords: Endometriosis; Laparoscopy; Pelvic pain; Randomised controlled trial; Surgical ablation; Surgical excision.
© 2023. The Author(s).
Conflict of interest statement
CMB has received research funding from Bayer Healthcare and has done consultancy for Myovant, ObsEva, Gedeon Richter and Theramex. YC has received honoraria for medical advisory from Merck, Ferring, Nordic Pharma and Abbot and research funding from Merck and Geurbet. AM has received funding to attend meetings and honoraria for speaking at educational events by Ferring, Merck, Cook Medical, Geodeon Ricter and Pharmasure. DCM has received legal expert witness fees from Lowenthal & Abrams, P.C. (Bala Cynwyd, PA) and funding to attend annual scientific meetings by EndoFound (Endometriosis Foundation of America) and the American Society of Reproductive Medicine (ASRM) as the 2022 recipient of the Society of Reproductive Surgeons (SRS) award recipient (a monetary award by the ASRM accompanied the SRS award). KV has received research funding from Bayer Healthcare and honoraria for consultancy for Bayer Healthcare, AbbVie, Eli Lilly and Reckitts. AWH has received honoraria for consultancy for Roche Diagnostics and Gesynta. AWH and LHRW have received research funding from Roche Diagnostics. SCM, KV, AWH and LHRW are listed as co-inventors on a UK Patent Application (No. 2217921.2) relating to the use of a genetic test to determine efficacy of gabapentin for chronic pelvic pain treatment. The other authors declare no conflict of interest pertaining to this manuscript.
Figures
References
-
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299. doi: 10.1093/humrep/des073. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous