Independent endothelial functions of PIEZO1 and TRPV4 in hepatic portal vein and predominance of PIEZO1 in mechanical and osmotic stress
- PMID: 37349903
- PMCID: PMC10946873
- DOI: 10.1111/liv.15646
Independent endothelial functions of PIEZO1 and TRPV4 in hepatic portal vein and predominance of PIEZO1 in mechanical and osmotic stress
Abstract
Background & aims: PIEZO1 and TRPV4 are mechanically and osmotically regulated calcium-permeable channels. The aim of this study was to determine the relevance and relationship of these channels in the contractile tone of the hepatic portal vein, which experiences mechanical and osmotic variations as it delivers blood to the liver from the intestines, gallbladder, pancreas and spleen.
Methods: Wall tension was measured in freshly dissected portal veins from adult male mice, which were genetically unmodified or modified for either a non-disruptive tag in native PIEZO1 or endothelial-specific PIEZO1 deletion. Pharmacological agents were used to activate or inhibit PIEZO1, TRPV4 and associated pathways, including Yoda1 and Yoda2 for PIEZO1 and GSK1016790A for TRPV4 agonism, respectively.
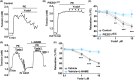
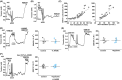
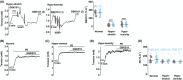
Results: PIEZO1 activation leads to nitric oxide synthase- and endothelium-dependent relaxation of the portal vein. TRPV4 activation causes contraction, which is also endothelium-dependent but independent of nitric oxide synthase. The TRPV4-mediated contraction is suppressed by inhibitors of phospholipase A2 and cyclooxygenases and mimicked by prostaglandin E2 , suggesting mediation by arachidonic acid metabolism. TRPV4 antagonism inhibits the effect of agonising TRPV4 but not PIEZO1. Increased wall stretch and hypo-osmolality inhibit TRPV4 responses while lacking effects on or amplifying PIEZO1 responses.
Conclusions: The portal vein contains independently functioning PIEZO1 channels and TRPV4 channels in the endothelium, the pharmacological activation of which leads to opposing effects of vessel relaxation (PIEZO1) and contraction (TRPV4). In mechanical and osmotic strain, the PIEZO1 mechanism dominates. Modulators of these channels could present important new opportunities for manipulating liver perfusion and regeneration in disease and surgical procedures.
Keywords: PIEZO channel; arachidonic acid metabolism; calcium signalling; calcium-permeable channel; endothelial nitric oxide synthase; endothelium; hepatobiliary system; liver; mechanical force; nitric oxide; non-selective cation channel; osmolality; portal vein; transient receptor potential vanilloid 4 (TRPV4) channel; vascular relaxation; vasculature contraction; vein.
© 2023 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest other than obligations from their research grants (Financial Support).
Figures



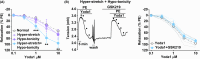
References
-
- Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress‐induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5(5):453‐464. - PubMed
-
- Takahashi S, Hitomi J, Satoh Y, Takahashi T, Asakura H, Ushiki T. Fine structure of the mouse portal vein in relation to its peristaltic movement. Arch Histol Cytol. 2002;65(1):71‐82. - PubMed
-
- Thievent A, Connat JL. Cytoskeletal features in longitudinal and circular smooth muscles during development of the rat portal vein. Cell Tissue Res. 1995;279(1):199‐208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

