Dynamics of urine proteomics biomarker and disease progression in patients with IgA nephropathy
- PMID: 37349951
- PMCID: PMC10689155
- DOI: 10.1093/ndt/gfad125
Dynamics of urine proteomics biomarker and disease progression in patients with IgA nephropathy
Abstract
Background: Immunoglobulin A nephropathy (IgAN) frequently leads to kidney failure. The urinary proteomics-based classifier IgAN237 may predict disease progression at the time of kidney biopsy. We studied whether IgAN237 also predicts progression later in the course of IgAN.
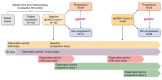
Methods: Urine from patients with biopsy-proven IgAN was analyzed using capillary electrophoresis-mass spectrometry at baseline (IgAN237-1, n = 103) and at follow-up (IgAN237-2, n = 89). Patients were categorized as "non-progressors" (IgAN237 ≤0.38) and "progressors" (IgAN237 >0.38). Estimated glomerular filtration rate (eGFR) and urinary albumin-creatinine ratio slopes were calculated.
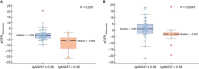
Results: Median age at biopsy was 44 years, interval between biopsy and IgAN237-1 was 65 months and interval between IgAN237-1 and IgAN237-2 was 258 days (interquartile range 71-531). IgAN237-1 and IgAN237-2 values did not differ significantly and were correlated (rho = 0.44, P < .001). Twenty-eight percent and 26% of patients were progressors based on IgAN237-1 and IgAN237-2, respectively. IgAN237 inversely correlated with chronic eGFR slopes (rho = -0.278, P = .02 for score-1; rho = -0.409, P = .002 for score-2) and with ±180 days eGFR slopes (rho = -0.31, P = .009 and rho = -0.439, P = .001, respectively). The ±180 days eGFR slopes were worse for progressors than for non-progressors (median -5.98 versus -1.22 mL/min/1.73 m2 per year for IgAN237-1, P < .001; -3.02 vs 1.08 mL/min/1.73 m2 per year for IgAN237-2, P = .0047). In multiple regression analysis baseline progressor/non-progressor according to IgAN237 was an independent predictor of eGFR180days-slope (P = .001).
Conclusion: The urinary IgAN237 classifier represents a risk stratification tool in IgAN also later in the course of the dynamic disease. It may guide patient management in an individualized manner.
Keywords: CKD; IgA nephropathy; biomarker; glomerulonephritis; progression; urine proteomics.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
J.S. is employed by Mosaiques Diagnostics GmbH. R.W. reports personal advisory and/or lecture fees from AstraZeneca, CSL Vifor and Boehringer Ingelheim outside the submitted work. A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma, and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes outside the submitted work. H.M. is the co-founder and co-owner of Mosaiques Diagnostics GmbH. H.N.R. reports other financial activities from Calliditas and from Omeros outside the submitted work. All other authors have nothing to disclose. The results presented in this article have not been published previously in whole or part, except in abstract format.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

