Newborn screening for Duchenne muscular dystrophy: A two-year pilot study
- PMID: 37350320
- PMCID: PMC10424650
- DOI: 10.1002/acn3.51829
Newborn screening for Duchenne muscular dystrophy: A two-year pilot study
Abstract
Objective: Duchenne muscular dystrophy (DMD) is an X-linked disorder resulting in progressive muscle weakness and atrophy, cardiomyopathy, and in late stages, cardiorespiratory impairment, and death. As treatments for DMD have expanded, a DMD newborn screening (NBS) pilot study was conducted in New York State to evaluate the feasibility and benefit of NBS for DMD and to provide an early pre-symptomatic diagnosis.
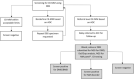
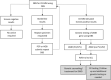
Methods: At participating hospitals, newborns were recruited to the pilot study, and consent was obtained to screen the newborn for DMD. The first-tier screen measured creatine kinase-MM (CK-MM) in dried blood spot specimens submitted for routine NBS. Newborns with elevated CK-MM were referred for genetic counseling and genetic testing. The latter included deletion/duplication analysis and next-generation sequencing (NGS) of the DMD gene followed by NGS for a panel of neuromuscular conditions if no pathogenic variants were detected in the DMD gene.
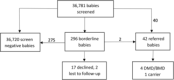
Results: In the two-year pilot study, 36,781 newborns were screened with CK-MM. Forty-two newborns (25 male and 17 female) were screen positive and referred for genetic testing. Deletions or duplications in the DMD gene were detected in four male infants consistent with DMD or Becker muscular dystrophy. One female DMD carrier was identified.
Interpretation: This study demonstrated that the state NBS program infrastructure and screening technologies we used are feasible to perform NBS for DMD. With an increasing number of treatment options, the clinical utility of early identification for affected newborns and their families lends support for NBS for this severe disease.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
W.K.C. is on the Board of Directors of Prime Medicine. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflict of interest.
Figures
References
-
- Mendell JR, Shilling C, Leslie ND, et al. Evidence‐based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304‐313. - PubMed
-
- Tuffery‐Giraud S, Béroud C, Leturcq F, et al. Genotype‐phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD‐DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009;30:934‐945. - PubMed
-
- Aartsma‐Rus A, Van Deutekom JC, Fokkema IF, et al. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading‐frame rule. Muscle Nerve. 2006;34:135‐144. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
