Polydeoxyribonucleotide exerts opposing effects on ERK activity in human skin keratinocytes and fibroblasts
- PMID: 37350391
- PMCID: PMC10308489
- DOI: 10.3892/mmr.2023.13035
Polydeoxyribonucleotide exerts opposing effects on ERK activity in human skin keratinocytes and fibroblasts
Abstract
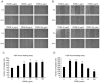
Polydeoxyribonucleotide (PDRN) is a mixture of deoxyribonucleotides. It serves as an anti‑inflammatory and tissue‑regenerating agent. The mitogen‑activated protein kinase pathway modulates cell growth and collagen accumulation. It also regulates inflammation by suppressing the expression of proinflammatory cytokines. In the present study, it was attempted to elucidate the molecular mechanism of PDRN in skin healing by confirming the effects of PDRN treatment on skin keratinocytes and fibroblasts, and by assessing the levels of collagen and inflammatory cytokines regulated by the extracellular signal‑regulated kinase (ERK) pathway. The potential effects of PDRN on skin regeneration were investigated. Fibroblast and keratinocyte proliferation and migration were analyzed using the water‑soluble tetrazolium‑8 and wound healing assays. The upregulation of collagen synthesis by PDRN‑induced ERK activation was analyzed in fibroblasts with or without an ERK inhibitor. Inflammatory cytokine expression levels in keratinocytes were determined using reverse transcription‑quantitative polymerase chain reaction. PDRN promoted the proliferation and migration of keratinocytes and fibroblasts. However, PDRN‑induced ERK phosphorylation differed between keratinocytes and fibroblasts; PDRN increased ERK phosphorylation and collagen accumulation in fibroblasts, while it inhibited matrix metalloproteinase expression. By contrast, PDRN inhibited ERK phosphorylation in keratinocytes, and it decreased inflammatory cytokine expression levels. PDRN affects skin cell proliferation and migration, and collagen and inflammatory cytokine expression levels via ERK signaling. Overall, PDRN exerts a positive effect on skin regeneration, but the mechanism by which it promotes skin regeneration varies among different skin cell types.
Keywords: extracellular signal‑regulated kinase; fibroblasts; keratinocytes; polydeoxyribonucleotide; skin regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

