Direct Differentiation of Bone Marrow Mononucleated Cells Into Insulin-Producing Cells Using 4 Specific Soluble Factors
- PMID: 37350544
- PMCID: PMC10346423
- DOI: 10.1093/stcltm/szad035
Direct Differentiation of Bone Marrow Mononucleated Cells Into Insulin-Producing Cells Using 4 Specific Soluble Factors
Abstract
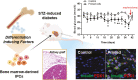
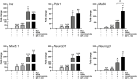
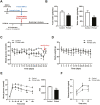
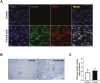
Bone marrow-derived stem cells are self-renewing and multipotent adult stem cells that differentiate into several types of cells. Here, we investigated a unique combination of 4 differentiation-inducing factors (DIFs), including putrescine (Put), glucosamine (GlcN), nicotinamide, and BP-1-102, to develop a differentiation method for inducing mature insulin-producing cells (IPCs) and apply this method to bone marrow mononucleated cells (BMNCs) isolated from mice. BMNCs, primed with the 4 soluble DIFs, were differentiated into functional IPCs. BMNCs cultured under the defined conditions synergistically expressed multiple genes, including those for PDX1, NKX6.1, MAFA, NEUROG3, GLUT2, and insulin, related to pancreatic beta cell development and function. They produced insulin/C-peptide and PDX1, as assessed using immunofluorescence and flow cytometry. The induced cells secreted insulin in a glucose-responsive manner, similar to normal pancreatic beta cells. Grafting BMNC-derived IPCs under kidney capsules of mice with streptozotocin (STZ)-induced diabetes alleviated hyperglycemia by lowering blood glucose levels, enhancing glucose tolerance, and improving glucose-stimulated insulin secretion. Insulin- and PDX1-expressing cells were observed in the IPC-bearing graft sections of nephrectomized mice. Therefore, this study provides a simple protocol for BMNC differentiation, which can be a novel approach for cell-based therapy in diabetes mellitus.
Keywords: a unique combination of differentiation-inducing factors; bone marrow mononucleated cells; differentiation; insulin-producing cells.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interests.
Figures


Similar articles
-
In Vivo Differentiation of Endogenous Bone Marrow-Derived Cells into Insulin-Producing Cells Using Four Soluble Factors.Diabetes Metab J. 2025 Jan;49(1):150-159. doi: 10.4093/dmj.2024.0174. Epub 2024 Oct 24. Diabetes Metab J. 2025. PMID: 39444334 Free PMC article.
-
Insulin-Producing Cells Differentiated from Human Bone Marrow Mesenchymal Stem Cells In Vitro Ameliorate Streptozotocin-Induced Diabetic Hyperglycemia.PLoS One. 2016 Jan 12;11(1):e0145838. doi: 10.1371/journal.pone.0145838. eCollection 2016. PLoS One. 2016. PMID: 26756576 Free PMC article.
-
Direct differentiation of bone marrow mononucleated cells into insulin producing cells using pancreatic β-cell-derived components.Sci Rep. 2019 Mar 29;9(1):5343. doi: 10.1038/s41598-019-41823-9. Sci Rep. 2019. PMID: 30926860 Free PMC article.
-
Pancreatic insulin-producing cells differentiated from human embryonic stem cells correct hyperglycemia in SCID/NOD mice, an animal model of diabetes.PLoS One. 2014 Jul 10;9(7):e102198. doi: 10.1371/journal.pone.0102198. eCollection 2014. PLoS One. 2014. PMID: 25009980 Free PMC article.
-
Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors.Int J Biochem Cell Biol. 2014 Jan;46:90-102. doi: 10.1016/j.biocel.2013.11.006. Epub 2013 Nov 22. Int J Biochem Cell Biol. 2014. PMID: 24275096 Review.
Cited by
-
The key regulation of LncRNA MALAT during reprogramming of primary mouse hepatocytes into insulin producing cells.Sci Rep. 2025 Jul 9;15(1):24614. doi: 10.1038/s41598-025-08106-y. Sci Rep. 2025. PMID: 40634357 Free PMC article.
-
A Supportive Role of Mesenchymal Stem Cells on Insulin-Producing Langerhans Islets with a Specific Emphasis on The Secretome.Biomedicines. 2023 Sep 18;11(9):2558. doi: 10.3390/biomedicines11092558. Biomedicines. 2023. PMID: 37761001 Free PMC article. Review.
-
Ameliorating and refining islet organoids to illuminate treatment and pathogenesis of diabetes mellitus.Stem Cell Res Ther. 2024 Jun 27;15(1):188. doi: 10.1186/s13287-024-03780-7. Stem Cell Res Ther. 2024. PMID: 38937834 Free PMC article. Review.
-
Low-intensity ultrasound stimulation promotes differentiation of bone marrow mononuclear cells to nucleus pulposus cells for matrix synthesis.Am J Transl Res. 2025 Feb 15;17(2):927-940. doi: 10.62347/LMPA6921. eCollection 2025. Am J Transl Res. 2025. PMID: 40092079 Free PMC article.
-
In Vivo Differentiation of Endogenous Bone Marrow-Derived Cells into Insulin-Producing Cells Using Four Soluble Factors.Diabetes Metab J. 2025 Jan;49(1):150-159. doi: 10.4093/dmj.2024.0174. Epub 2024 Oct 24. Diabetes Metab J. 2025. PMID: 39444334 Free PMC article.

