Targeted memory reactivation in human REM sleep elicits detectable reactivation
- PMID: 37350572
- PMCID: PMC10425171
- DOI: 10.7554/eLife.84324
Targeted memory reactivation in human REM sleep elicits detectable reactivation
Abstract
It is now well established that memories can reactivate during non-rapid eye movement (non-REM) sleep, but the question of whether equivalent reactivation can be detected in rapid eye movement (REM) sleep is hotly debated. To examine this, we used a technique called targeted memory reactivation (TMR) in which sounds are paired with learned material in wake, and then re-presented in subsequent sleep, in this case REM, to trigger reactivation. We then used machine learning classifiers to identify reactivation of task-related motor imagery from wake in REM sleep. Interestingly, the strength of measured reactivation positively predicted overnight performance improvement. These findings provide the first evidence for memory reactivation in human REM sleep after TMR that is directly related to brain activity during wakeful task performance.
Keywords: REM sleep; human; memory reactivation; neuroscience; targeted memory reactivation.
Plain language summary
Sleep is crucial for rest and recovery, but it also allows the brain to process things it has learned while awake. This is why a person may go to bed frustrated with learning a tune on the piano but wake up the next morning ready to play it without fumbling. For this to happen, it is thought that memories must be reactivated during sleep – something which can be studied by monitoring brain activity. While it has been shown that memory reactivation occurs in some stages of human sleep, it was unclear whether it occurred in a specific stage known as REM sleep – which is important for learning. To study memory reactivation during REM sleep, Abdellahi et al. recruited volunteers and monitored their brain activity during an ‘adaptation night’ when certain sounds played as they slept. The following day, memories – such as an image or pressing a certain button – were paired with the sounds, which were replayed during REM sleep the following night to trigger memory reactivation (experimental night). Abdellahi et al. measured how strongly brain activity during each night related to the waking activity when the sound pairing tasks were imagined and compared the adaptation and experimental nights. The experimental night showed clear signs of memory reactivation after the sounds were played during REM sleep, suggesting that the sounds triggered memories of the associated images or buttons. These findings show that in humans, brain activity patterns that indicate memory reactivation can be identified during REM sleep. The work paves the way for future studies into the characteristics of this memory reactivation and how to trigger it in a way that leads to improvements in memory.
© 2023, Abdellahi et al.
Conflict of interest statement
MA, AK, MT, PL No competing interests declared
Figures
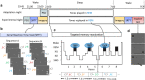

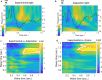
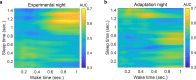
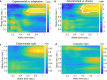



Update of
References
-
- Abdellahi ME. Lively vectors. GitHub. 2022 https://github.com/MahmoudAbdellahi/Lively_Vectors
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

