Aldehyde accumulation in Mycobacterium tuberculosis with defective proteasomal degradation results in copper sensitivity
- PMID: 37350636
- PMCID: PMC10470581
- DOI: 10.1128/mbio.00363-23
Aldehyde accumulation in Mycobacterium tuberculosis with defective proteasomal degradation results in copper sensitivity
Abstract
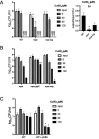
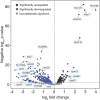
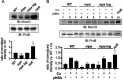
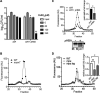
Mycobacterium tuberculosis is a major human pathogen and the causative agent of tuberculosis disease. M. tuberculosis is able to persist in the face of host-derived antimicrobial molecules nitric oxide (NO) and copper (Cu). However, M. tuberculosis with defective proteasome activity is highly sensitive to NO and Cu, making the proteasome an attractive target for drug development. Previous work linked NO susceptibility with the accumulation of para-hydroxybenzaldehyde (pHBA) in M. tuberculosis mutants with defective proteasomal degradation. In this study, we found that pHBA accumulation was also responsible for Cu sensitivity in these strains. We showed that exogenous addition of pHBA to wild-type M. tuberculosis cultures sensitized bacteria to Cu to a degree similar to that of a proteasomal degradation mutant. We determined that pHBA reduced the production and function of critical Cu resistance proteins of the
Keywords: Mycobacterium tuberculosis; aldehyde; copper; proteasome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The copper-responsive RicR regulon contributes to Mycobacterium tuberculosis virulence.mBio. 2014 Feb 18;5(1):e00876-13. doi: 10.1128/mBio.00876-13. mBio. 2014. PMID: 24549843 Free PMC article.
-
Proteasomal control of cytokinin synthesis protects Mycobacterium tuberculosis against nitric oxide.Mol Cell. 2015 Mar 19;57(6):984-994. doi: 10.1016/j.molcel.2015.01.024. Epub 2015 Feb 26. Mol Cell. 2015. PMID: 25728768 Free PMC article.
-
Diisonitrile Lipopeptides Mediate Resistance to Copper Starvation in Pathogenic Mycobacteria.mBio. 2022 Oct 26;13(5):e0251322. doi: 10.1128/mbio.02513-22. Epub 2022 Oct 5. mBio. 2022. PMID: 36197089 Free PMC article.
-
The aldehyde hypothesis: metabolic intermediates as antimicrobial effectors.Open Biol. 2022 Apr;12(4):220010. doi: 10.1098/rsob.220010. Epub 2022 Apr 13. Open Biol. 2022. PMID: 35414258 Free PMC article. Review.
-
Copper homeostasis in Mycobacterium tuberculosis.Metallomics. 2015 Jun;7(6):929-34. doi: 10.1039/c4mt00305e. Metallomics. 2015. PMID: 25614981 Free PMC article. Review.
Cited by
-
Identification of glyoxalase A in group B Streptococcus and its contribution to methylglyoxal tolerance and virulence.Infect Immun. 2025 Apr 8;93(4):e0054024. doi: 10.1128/iai.00540-24. Epub 2025 Feb 26. Infect Immun. 2025. PMID: 40008888 Free PMC article.
-
A glyoxal-specific aldehyde signaling axis in Pseudomonas aeruginosa that influences quorum sensing and infection.Nat Commun. 2025 Jul 18;16(1):6616. doi: 10.1038/s41467-025-61469-8. Nat Commun. 2025. PMID: 40681494 Free PMC article.
-
The efflux pumps Rv1877 and Rv0191 play differential roles in the protection of Mycobacterium tuberculosis against chemical stress.Front Microbiol. 2024 Mar 4;15:1359188. doi: 10.3389/fmicb.2024.1359188. eCollection 2024. Front Microbiol. 2024. PMID: 38516013 Free PMC article.
-
Identification of Glyoxalase A in Group B Streptococcus and its contribution to methylglyoxal tolerance and virulence.bioRxiv [Preprint]. 2024 Dec 19:2024.07.30.605887. doi: 10.1101/2024.07.30.605887. bioRxiv. 2024. Update in: Infect Immun. 2025 Apr 08;93(4):e0054024. doi: 10.1128/iai.00540-24. PMID: 39131367 Free PMC article. Updated. Preprint.
-
Characterization of a cytokinin-binding protein locus in Mycobacterium tuberculosis.J Bacteriol. 2025 Mar 20;207(3):e0000325. doi: 10.1128/jb.00003-25. Epub 2025 Feb 27. J Bacteriol. 2025. PMID: 40013803 Free PMC article.
References
-
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudget JS. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641–650. doi:10.1016/0092-8674(95)90085-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

