Preexisting Immunity Drives the Response to Neoadjuvant Chemotherapy in Esophageal Adenocarcinoma
- PMID: 37350667
- PMCID: PMC10472105
- DOI: 10.1158/0008-5472.CAN-23-0356
Preexisting Immunity Drives the Response to Neoadjuvant Chemotherapy in Esophageal Adenocarcinoma
Abstract
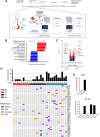
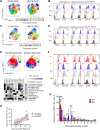
Current treatment for patients with locally advanced esophageal adenocarcinoma (EAC) is neoadjuvant chemotherapy (nCT), alone or combined with radiotherapy, before surgery. However, fewer than 30% of treated patients show a pathologic complete response to nCT, which correlates with increased 5-year survival compared with nonresponders. Understanding the mechanisms of response to nCT is pivotal to better stratify patients and inform more efficacious therapies. Here, we investigated the immune mechanisms involved in nCT response by multidimensional profiling of pretreatment tumor biopsies and blood from 68 patients with EAC (34 prospectively and 34 retrospectively collected), comparing complete responders versus nonresponders to nCT. At the tumor level, complete response to nCT was associated with molecular signatures of immune response and proliferation, increased putative antitumor tissue-resident memory CD39+ CD103+ CD8+ T cells, and reduced immunosuppressive T regulatory cells (Treg) and M2-like macrophages. Systemically, complete responders showed higher frequencies of immunostimulatory CD14+ CD11c+ HLA-DRhigh cells, and reduced programmed cell death ligand 1-positive (PD-L1+) monocytic myeloid-derived suppressor cells, along with high plasma GM-CSF (proinflammatory) and low IL4, CXCL10, C3a, and C5a (suppressive). Plasma proinflammatory and suppressive cytokines correlated directly and inversely, respectively, with the frequency of tumor-infiltrating CD39+ CD103+ CD8+ T cells. These results suggest that preexisting immunity in baseline tumor drives the clinical activity of nCT in locally advanced EAC. Furthermore, it may be possible to stratify patients based on predictive immune signatures, enabling tailored neoadjuvant and/or adjuvant regimens.
Significance: Multidimensional profiling of pretreatment esophageal adenocarcinoma shows patient response to nCT is correlated with active preexisting immunity and indicates molecular pathways of resistance that may be targeted to improve clinical outcomes.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures






References
-
- Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. . Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–8. - PubMed
-
- Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. . Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:992–1004. - PubMed
-
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

