Histological validation of atrial structural remodelling in patients with atrial fibrillation
- PMID: 37350738
- PMCID: PMC10499545
- DOI: 10.1093/eurheartj/ehad396
Histological validation of atrial structural remodelling in patients with atrial fibrillation
Abstract
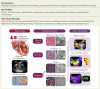
Background and aims: This study aimed to histologically validate atrial structural remodelling associated with atrial fibrillation.
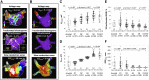
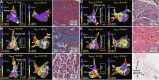
Methods and results: Patients undergoing atrial fibrillation ablation and endomyocardial atrial biopsy were included (n = 230; 67 ± 12 years old; 69 women). Electroanatomic mapping was performed during right atrial pacing. Voltage at the biopsy site (Vbiopsy), global left atrial voltage (VGLA), and the proportion of points with fractionated electrograms defined as ≥5 deflections in each electrogram (%Fractionated EGM) were evaluated. SCZtotal was calculated as the total width of slow conduction zones, defined as regions with a conduction velocity of <30 cm/s. Histological factors potentially associated with electroanatomic characteristics were evaluated using multiple linear regression analyses. Ultrastructural features and immune cell infiltration were evaluated by electron microscopy and immunohistochemical staining in 33 and 60 patients, respectively. Fibrosis, intercellular space, myofibrillar loss, and myocardial nuclear density were significantly associated with Vbiopsy (P = .014, P < .001, P < .001, and P = .002, respectively) and VGLA (P = .010, P < .001, P = .001, and P < .001, respectively). The intercellular space was associated with the %Fractionated EGM (P = .001). Fibrosis, intercellular space, and myofibrillar loss were associated with SCZtotal (P = .028, P < .001, and P = .015, respectively). Electron microscopy confirmed plasma components and immature collagen fibrils in the increased intercellular space and myofilament lysis in cardiomyocytes, depending on myofibrillar loss. Among the histological factors, the severity of myofibrillar loss was associated with an increase in macrophage infiltration.
Conclusion: Histological correlates of atrial structural remodelling were fibrosis, increased intercellular space, myofibrillar loss, and decreased nuclear density. Each histological component was defined using electron microscopy and immunohistochemistry studies.
Keywords: Atrial biopsy; Atrial fibrillation; Electroanatomic mapping; Fibrosis; Histology; Structural remodelling.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




Comment in
-
Histopathology of atrial fibrillation: does this help us understand its pathogenesis?Eur Heart J. 2023 Sep 14;44(35):3354-3356. doi: 10.1093/eurheartj/ehad515. Eur Heart J. 2023. PMID: 37590445 No abstract available.
References
-
- Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, et al. . Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol 2003;42:185–197. 10.1016/S0735-1097(03)00577-1 - DOI - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

