Cr(vi) permanently binds to the lipid bilayer in an inverted hexagonal phase throughout the reduction process
- PMID: 37350866
- PMCID: PMC10282592
- DOI: 10.1039/d2ra07851a
Cr(vi) permanently binds to the lipid bilayer in an inverted hexagonal phase throughout the reduction process
Abstract
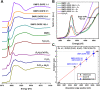
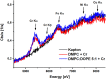
Cr(vi) is a harmful, carcinogenic agent with a high permeability rate throughout the lipid membranes. In an intracellular environment and during interactions with cellular membranes, it undergoes an instant reduction to lower oxidation states throughout radical states, recognized as the most dangerous factor for cells. The cellular membrane is the most visible cellular organelle in the interior and exterior of a cell. In this study, liposomes and non-lamellar inverted hexagonal phase lipid structures based on phosphoethanolamine (PE) were used as model cellular bilayers because of their simple composition, preparation procedure, and the many other properties of natural systems. The lipid membranes were subjected to 0.075 mM Cr(vi) for 15 min, after which the Cr content was removed via dialysis. This way, the remaining Cr content could be studied qualitatively and quantitatively. Using the combined XRF/XAS/EPR approach, we revealed that some Cr content (Cr(iii) and Cr(vi)) was still present in the samples even after long-term dialysis at a temperature significantly above the phase transition for the chosen liposome. The amount of bound Cr increased with increasing PE and -C[double bond, length as m-dash]C- bond content in lipid mixtures. Internal membrane order decreased in less fluid membranes, while in more liquified ones, internal order was only slightly changed after subjecting them to the Cr(vi) agent. The results suggest that the inverted hexagonal phase of lipid structures is much more sensitive to oxidation than the lamellar lipid phase, which can play an important role in the strong cytotoxicity of Cr(vi).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion.Biophys J. 1986 Jun;49(6):1171-83. doi: 10.1016/S0006-3495(86)83745-6. Biophys J. 1986. PMID: 3719075 Free PMC article.
-
Lipid dependence of diadinoxanthin solubilization and de-epoxidation in artificial membrane systems resembling the lipid composition of the natural thylakoid membrane.Biochim Biophys Acta. 2007 Jan;1768(1):67-75. doi: 10.1016/j.bbamem.2006.06.006. Epub 2006 Jun 7. Biochim Biophys Acta. 2007. PMID: 16843433
-
Implications of a non-lamellar lipid phase for the tight junction stability. Part I: Influence of basic amino acids, pH and protamine on the bilayer-hexagonal II phase behaviour of PS-containing PE membranes.Chem Phys Lipids. 1992 Dec;63(3):213-21. doi: 10.1016/0009-3084(92)90037-p. Chem Phys Lipids. 1992. PMID: 1493615
-
A lipid-phase separation model of low-temperature damage to biological membranes.Cryobiology. 1985 Apr;22(2):128-46. doi: 10.1016/0011-2240(85)90167-1. Cryobiology. 1985. PMID: 3920005 Review.
-
Structure and functional properties of diacylglycerols in membranes.Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review.
References
-
- Mulyani I. Levina A. Lay P. A. Angew. Chem., Int. Ed. 2004;43:4504–4507. - PubMed
-
- Levina A. and Lay P. A., The Nutritional Biochemistry of Chromium (III), Elsevier, 2019, pp. 281–321
-
- Speetjens J. K. Collins R. A. Vincent J. B. Woski S. A. Chem. Res. Toxicol. 1999;12:483–487. - PubMed
-
- Farkas N. Pesti M. Belagyi J. Biochim. Biophys. Acta, Biomembr. 2003;1611:217–222. - PubMed
LinkOut - more resources
Full Text Sources

