Endothelial glycocalyx injury is involved in heatstroke-associated coagulopathy and protected by N-acetylcysteine
- PMID: 37350963
- PMCID: PMC10283401
- DOI: 10.3389/fimmu.2023.1159195
Endothelial glycocalyx injury is involved in heatstroke-associated coagulopathy and protected by N-acetylcysteine
Abstract
Introduction: Damage to endothelial glycocalyx (EGCX) can lead to coagulation disorders in sepsis. Heat stroke (HS) resembles sepsis in many aspects; however, it is unclear whether EGCX injury is involved in its pathophysiology. The purpose of this study was to examine the relationship between the damage of EGCX and the development of coagulation disorders during HS.
Methods: We retrospectively collected 159 HS patients and analyzed coagulation characteristics and prognosis of HS patients with or without disseminated intravascular coagulation (DIC). We also replicated a rat HS model and measured coagulation indexes, pulmonary capillary EGCX injury in HS rats. Finally, we evaluated the effect of the antioxidant N-acetylcysteine (NAC) on HS-initiated EGCX injury and coagulation disorders.
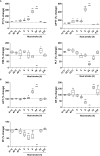
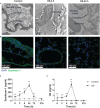
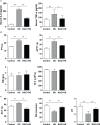
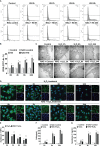
Results: Clinical data showed that HS patients complicated with DIC had a higher risk of death than HS patients without DIC. In a rat HS model, we found that rats subjected to heat stress developed hypercoagulability and platelet activation at the core body temperature of 43°C, just before the onset of HS. At 24 h of HS, the rats showed a consumptive hypo-coagulation state. The pulmonary capillary EGCX started to shed at 0 h of HS and became more severe at 24 h of HS. Importantly, pretreatment with NAC substantially alleviated EGCX damage and reversed the hypo-coagulation state in HS rats. Mechanically, HS initiated reactive oxidative species (ROS) generation, while ROS could directly cause EGCX damage. Critically, NAC protected against EGCX injury by attenuating ROS production in heat-stressed or hydrogen peroxide (H2O2)-stimulated endothelial cells.
Discussion: Our results indicate that the poor prognosis of HS patients correlates with severe coagulation disorders, coagulation abnormalities in HS rats are associated with the damage of EGCX, and NAC improves HS-induced coagulopathy, probably through its protection against EGCX injury by preventing ROS generation.
Keywords: N-acetylcysteine; coagulopathy; endothelial glycocalyx; heat stroke; hyaluronic acid; syndecan-1.
Copyright © 2023 Peng, Geng, Ouyang, Liu, Yuan, Wan, Chen, Yu, Tang, Su, Liang, Wang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Heat stress combined with lipopolysaccharide induces pulmonary microvascular endothelial cell glycocalyx inflammatory damage in vitro.Immun Inflamm Dis. 2023 Oct;11(10):e1034. doi: 10.1002/iid3.1034. Immun Inflamm Dis. 2023. PMID: 37904703 Free PMC article.
-
Dexmedetomidine preserves the endothelial glycocalyx and improves survival in a rat heatstroke model.J Anesth. 2018 Dec;32(6):880-885. doi: 10.1007/s00540-018-2568-7. Epub 2018 Oct 29. J Anesth. 2018. PMID: 30374889
-
Sodium tanshinone IIA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model.Mol Med Rep. 2017 Jul;16(1):87-94. doi: 10.3892/mmr.2017.6573. Epub 2017 May 11. Mol Med Rep. 2017. PMID: 28498471 Free PMC article.
-
Focus of endothelial glycocalyx dysfunction in ischemic stroke and Alzheimer's disease: Possible intervention strategies.Ageing Res Rev. 2024 Aug;99:102362. doi: 10.1016/j.arr.2024.102362. Epub 2024 Jun 1. Ageing Res Rev. 2024. PMID: 38830545 Review.
-
The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis.Front Cell Dev Biol. 2021 Jul 14;9:711003. doi: 10.3389/fcell.2021.711003. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34336864 Free PMC article. Review.
Cited by
-
High Glucose Induces Oxidative Stress That Alters Glycocalyx Proteoglycan Levels in Primary Rat Retinal Microvascular Endothelial Cells and in Isolated Ophthalmic Arteries.Pathophysiology. 2024 Feb 6;31(1):89-99. doi: 10.3390/pathophysiology31010007. Pathophysiology. 2024. PMID: 38390944 Free PMC article.
-
Impact of hyper- and hypothermia on cellular and whole-body physiology.J Intensive Care. 2025 Jan 13;13(1):4. doi: 10.1186/s40560-024-00774-8. J Intensive Care. 2025. PMID: 39806520 Free PMC article. Review.
-
The pathogenesis and therapeutic strategies of heat stroke-induced endothelial injury.Front Cell Dev Biol. 2025 Jul 9;13:1569346. doi: 10.3389/fcell.2025.1569346. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40703654 Free PMC article. Review.
-
How can heatstroke damage the brain? A mini review.Front Neurosci. 2024 Oct 10;18:1437216. doi: 10.3389/fnins.2024.1437216. eCollection 2024. Front Neurosci. 2024. PMID: 39450121 Free PMC article. Review.
-
The pathogenesis and management of heatstroke and heatstroke-induced lung injury.Burns Trauma. 2025 Jan 14;13:tkae048. doi: 10.1093/burnst/tkae048. eCollection 2025. Burns Trauma. 2025. PMID: 39811431 Free PMC article. Review.
References
-
- Misset B, De Jonghe B, Bastuji-Garin S, Gattolliat O, Boughrara E, Annane D, et al. . Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a national multiple-center risk-factor study. Crit Care Med (2006) 34(4):1087–92. doi: 10.1097/01.CCM.0000206469.33615.02 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

