Incorporating lesion-to-lesion heterogeneity into early oncology decision making
- PMID: 37350966
- PMCID: PMC10282604
- DOI: 10.3389/fimmu.2023.1173546
Incorporating lesion-to-lesion heterogeneity into early oncology decision making
Abstract
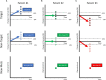
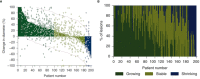
RECISTv1.1 (Response Evaluation Criteria In Solid Tumors) is the most commonly used response grading criteria in early oncology trials. In this perspective, we argue that RECISTv1.1 is ambiguous regarding lesion-to-lesion variation that can introduce bias in decision making. We show theoretical examples of how lesion-to-lesion variability causes bias in RECISTv1.1, leading to misclassification of patient response. Next, we review immune checkpoint inhibitor (ICI) clinical trial data and find that lesion-to-lesion heterogeneity is widespread in ICI-treated patients. We illustrate the implications of ignoring lesion-to-lesion heterogeneity in interpreting biomarker data, selecting treatments for patients with progressive disease, and go/no-go decisions in drug development. Further, we propose that Quantitative Systems Pharmacology (QSP) models can aid in developing better metrics of patient response and treatment efficacy by capturing patient responses robustly by considering lesion-to-lesion heterogeneity. Overall, we believe patient response evaluation with an appreciation of lesion-to-lesion heterogeneity can potentially improve decision-making at the early stage of oncology drug development and benefit patient care.
Keywords: QSP model; RECIST v1.1; dissociated response; lesion-to-lesion heterogeneity; oncology clinical trials.
Copyright © 2023 Kumar, Qi, Cao and Topp.
Conflict of interest statement
Author BT reports employment at the company Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, United States, and is a shareholder in Merck & Co., Inc., Kenilworth, NJ, United States. Author RK was employed by the company Vantage Research Inc. Vantage Research was engaged by MSD as a Contract Research Organization. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


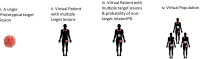

Similar articles
-
RECISTv1.1 progression in oncology: Shades of gray.Cancer Cell. 2023 Jun 12;41(6):1003-1005. doi: 10.1016/j.ccell.2023.04.012. Epub 2023 May 11. Cancer Cell. 2023. PMID: 37172579
-
Utilization of target lesion heterogeneity for treatment efficacy assessment in late stage lung cancer.PLoS One. 2021 Jul 1;16(7):e0252041. doi: 10.1371/journal.pone.0252041. eCollection 2021. PLoS One. 2021. PMID: 34197475 Free PMC article.
-
Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment.Abdom Radiol (NY). 2019 Feb;44(2):766-774. doi: 10.1007/s00261-018-1752-4. Abdom Radiol (NY). 2019. PMID: 30196362 Free PMC article. Review.
-
A multistate model for early decision-making in oncology.Biom J. 2020 May;62(3):550-567. doi: 10.1002/bimj.201800250. Epub 2019 Jul 16. Biom J. 2020. PMID: 31310368
-
Immune Response Evaluation and Treatment with Immune Checkpoint Inhibitors Beyond Clinical Progression: Response Assessments for Cancer Immunotherapy.Curr Oncol Rep. 2020 Aug 27;22(11):116. doi: 10.1007/s11912-020-00974-z. Curr Oncol Rep. 2020. PMID: 32851542 Review.
Cited by
-
An integrated quantitative systems pharmacology virtual population approach for calibration with oncology efficacy endpoints.CPT Pharmacometrics Syst Pharmacol. 2025 Feb;14(2):268-278. doi: 10.1002/psp4.13270. Epub 2024 Nov 7. CPT Pharmacometrics Syst Pharmacol. 2025. PMID: 39508122 Free PMC article.
-
Virtual patient analysis identifies strategies to improve the performance of predictive biomarkers for PD-1 blockade.Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2410911121. doi: 10.1073/pnas.2410911121. Epub 2024 Oct 28. Proc Natl Acad Sci U S A. 2024. PMID: 39467131 Free PMC article.
-
Eliciting the antitumor immune response with a conditionally activated PD-L1 targeting antibody analyzed with a quantitative systems pharmacology model.CPT Pharmacometrics Syst Pharmacol. 2024 Jan;13(1):93-105. doi: 10.1002/psp4.13060. Epub 2023 Dec 7. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38058278 Free PMC article.
-
Dissection of Progressive Disease Patterns for a Modified Classification for Immunotherapy.JAMA Oncol. 2025 Feb 1;11(2):154-161. doi: 10.1001/jamaoncol.2024.5672. JAMA Oncol. 2025. PMID: 39724246
-
Evaluating the tumor response assessment of modified-RECIST 1.1 in advance non-small cell lung cancer: a post-hoc analysis of 1,147 patients from the EAST-LC trial.Transl Lung Cancer Res. 2025 May 30;14(5):1688-1698. doi: 10.21037/tlcr-24-809. Epub 2025 May 23. Transl Lung Cancer Res. 2025. PMID: 40535076 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

