Hypofractionated radiotherapy with immunochemotherapy for extensive-stage small-cell lung cancer
- PMID: 37350968
- PMCID: PMC10282832
- DOI: 10.3389/fimmu.2023.1175960
Hypofractionated radiotherapy with immunochemotherapy for extensive-stage small-cell lung cancer
Abstract
Introduction: The combination of a PD-L1 inhibitor plus carboplatin/cisplatin and etoposide (EC/EP) has become a new standard first-line treatment for extensive-stage small-cell lung cancer (ES-SCLC). Combining concurrent palliative hypofractionated radiotherapy of the thorax (HFRT) and immunochemotherapy may have a synergistic effect. In this study, we explored an optimal model of combination radiotherapy with immunochemotherapy as first-line treatment of ES-SCLC.
Patients and methods: In this multicenter single-arm phase 2 trial, patients with ES-SCLC received atezolizumab with EC/EP for two cycles (induction phase), then, those who did not progress received concurrent palliative HFRT and two cycles of atezolizumab with EC/EP (combination phase). Afterward they received atezolizumab every 3 weeks for a maximum of 2 years after study enrolment (maintenance phase). Prophylactic cranial irradiation (PCI) was recommended. The primary endpoints were safety and tolerance; the second endpoints were progression-free survival (PFS).
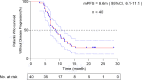
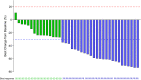
Results: Forty patients were enrolled, and all had completed palliative HFRT and four cycles of immunochemotherapy. There were seven grade 3 adverse events (3 decreased neutrophil count, 1 anemia, 2 pneumonitis, 1 esoenteritis), two grade 4 adverse events (2 decreased white cell count) and no grade 5 toxicities. The pneumonitis rate was 12.5% (three grade 2 and two grade 3 events). At the median follow-up of 14.2 months (range, 6.8-28.7), the median PFS was 8.6 months (95%CI, 6.1-11.1).
Conclusion: The addition of concurrent hypofractionated thoracic radiotherapy to first-line immunochemotherapy for ES-SCLC was well tolerated and showed promising clinical efficacy. Additional randomized trials are needed to validate benefits.
Clinical trial registration: https://clinicaltrials.gov/ (NCT04636762).
Keywords: extensive-stage small-cell lung cancer; immunochemotherapy; progression free survival; safety; thoracic radiation.
Copyright © 2023 Liu, Zeng, Deng, Jiang, Wang, Zhou, Liu, Wang, Zhou, Qiu, Zeng, Wu, Weng, Liu, Yang and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Carboplatin, etoposide, atezolizumab, and bevacizumab in the first-line treatment of patients with extensive stage small-cell lung cancer: the GOIRC-01-2019 CeLEBrATE study.J Immunother Cancer. 2025 May 7;13(5):e010694. doi: 10.1136/jitc-2024-010694. J Immunother Cancer. 2025. PMID: 40341031 Free PMC article. Clinical Trial.
-
Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial.Lancet Oncol. 2021 Jan;22(1):51-65. doi: 10.1016/S1470-2045(20)30539-8. Epub 2020 Dec 4. Lancet Oncol. 2021. PMID: 33285097 Clinical Trial.
-
Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial.Nat Med. 2024 Oct;30(10):2967-2976. doi: 10.1038/s41591-024-03132-1. Epub 2024 Jul 11. Nat Med. 2024. PMID: 38992123 Free PMC article. Clinical Trial.
-
Durvalumab: A Review in Extensive-Stage SCLC.Target Oncol. 2021 Nov;16(6):857-864. doi: 10.1007/s11523-021-00843-0. Epub 2021 Nov 3. Target Oncol. 2021. PMID: 34731446 Free PMC article. Review.
-
Atezolizumab: A Review in Extensive-Stage SCLC.Drugs. 2020 Oct;80(15):1587-1594. doi: 10.1007/s40265-020-01398-6. Drugs. 2020. PMID: 32990939 Review.
Cited by
-
Local radiotherapy in extensive-stage small-cell lung cancer sustainably boosts the clinical benefit of first-line immunotherapy: a case report.Front Immunol. 2024 Nov 1;15:1493740. doi: 10.3389/fimmu.2024.1493740. eCollection 2024. Front Immunol. 2024. PMID: 39555071 Free PMC article.
-
Real-world evaluation of first-line treatment of extensive-stage small-cell lung cancer with atezolizumab plus platinum/etoposide: a focus on patients with brain metastasis.Clin Transl Oncol. 2024 Jul;26(7):1664-1673. doi: 10.1007/s12094-024-03387-7. Epub 2024 Feb 8. Clin Transl Oncol. 2024. PMID: 38329610
-
The Optimal Radiotherapy Strategy for Patients With Small Cell Lung Cancer and Brain Metastasis: A Retrospective Analysis.CNS Neurosci Ther. 2024 Nov;30(11):e70102. doi: 10.1111/cns.70102. CNS Neurosci Ther. 2024. PMID: 39500635 Free PMC article.
-
Long-term survival after combination therapy with atezolizumab in a patient with small-cell lung cancer: a case report.Transl Lung Cancer Res. 2024 Dec 31;13(12):3795-3806. doi: 10.21037/tlcr-24-981. Epub 2024 Dec 26. Transl Lung Cancer Res. 2024. PMID: 39830746 Free PMC article.
-
Early radiotherapy improved survival of patients with extensive-stage small cell lung cancer treated with first-line chemo-immunotherapy.BMC Cancer. 2025 Jun 6;25(1):1012. doi: 10.1186/s12885-025-14417-0. BMC Cancer. 2025. PMID: 40481435 Free PMC article.
References
-
- Owonikoko TK, Dahlberg SE, Sica GL, Wagner LI, Wade JL, 3rd, Srkalovic G, et al. . Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small-cell lung cancer: ECOG-ACRIN 2511 study. J Clin Oncol (2019) 37:222–229. doi: 10.1200/JCO.18.00264 - DOI - PMC - PubMed
-
- Seckl MJ, Ottensmeier CH, Cullen M, Schmid P, Ngai Y, Muthukumar D, et al. . Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (LUNGSTAR). J Clin Oncol (2017) 35:1506–14. doi: 10.1200/JCO.2016.69.7391 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

