Shear stress regulation of nanoparticle uptake in vascular endothelial cells
- PMID: 37351014
- PMCID: PMC10281962
- DOI: 10.1093/rb/rbad047
Shear stress regulation of nanoparticle uptake in vascular endothelial cells
Abstract
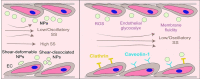
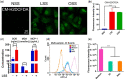
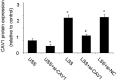
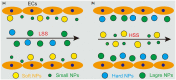
Nanoparticles (NPs) hold tremendous targeting potential in cardiovascular disease and regenerative medicine, and exciting clinical applications are coming into light. Vascular endothelial cells (ECs) exposure to different magnitudes and patterns of shear stress (SS) generated by blood flow could engulf NPs in the blood. However, an unclear understanding of the role of SS on NP uptake is hindering the progress in improving the targeting of NP therapies. Here, the temporal and spatial distribution of SS in vascular ECs and the effect of different SS on NP uptake in ECs are highlighted. The mechanism of SS affecting NP uptake through regulating the cellular ROS level, endothelial glycocalyx and membrane fluidity is summarized, and the molecules containing clathrin and caveolin in the engulfment process are elucidated. SS targeting NPs are expected to overcome the current bottlenecks and change the field of targeting nanomedicine. This assessment on how SS affects the cell uptake of NPs and the marginalization of NPs in blood vessels could guide future research in cell biology and vascular targeting drugs.
Keywords: caveolin; clathrin; endothelial cell; nanoparticle uptake; shear stress.
© The Author(s) 2023. Published by Oxford University Press.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

