Mating modifies the expression of crucial oxidative-reductive transcripts in the pig oviductal sperm reservoir: is the female ensuring sperm survival?
- PMID: 37351104
- PMCID: PMC10282951
- DOI: 10.3389/fendo.2023.1042176
Mating modifies the expression of crucial oxidative-reductive transcripts in the pig oviductal sperm reservoir: is the female ensuring sperm survival?
Abstract
Background: Mating induces large changes in the female genital tract, warranting female homeostasis and immune preparation for pregnancy, including the preservation of crucial oxidative status among its pathways. Being highly susceptible to oxidative stress, sperm survival and preserved function depend on the seminal plasma, a protection that is removed during sperm handling but also after mating when spermatozoa enter the oviduct. Therefore, it is pertinent to consider that the female sperm reservoir takes up this protection, providing a suitable environment for sperm viability. These aspects have not been explored despite the increasing strategies in modulating the female status through diet control and nutritional supplementation.
Aims: To test the hypothesis that mating modifies the expression of crucial oxidative-reductive transcripts across the entire pig female genital tract (cervix to infundibulum) and, particularly in the sperm reservoir at the utero-tubal junction, before ovulation, a period dominated by estrogen stimulation of ovarian as well as of seminal origin.
Methods: The differential expression of estrogen (ER) and progesterone (PR) receptors and of 59 oxidative-reductive transcripts were studied using a species-specific microarray platform, in specific segments of the peri-ovulatory sow reproductive tract in response to mating.
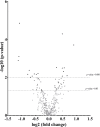
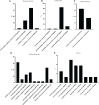
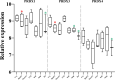
Results: Mating induced changes along the entire tract, with a conspicuous downregulation of both ER and PR and an upregulation of superoxide dismutase 1 (SOD1), glutaredoxin (GLRX3), and peroxiredoxin 1 and 3 (PRDX1, PRDX3), among other NADH Dehydrogenase Ubiquinone Flavoproteins, in the distal uterus segment. These changes perhaps helped prevent oxidative stress in the area adjacent to the sperm reservoir at the utero-tubal junction. Concomitantly, there were a downregulation of catalase (CAT) and NADH dehydrogenase (ubiquinone) oxidoreductases 1 beta subcomplex, subunit 1 (NDUFB1) in the utero-tubal junction alongside an overall downregulation of CAT, SOD1, and PRDX3 in the ampullar and infundibulum segments.
Conclusions: Natural mating is an inducer of changes in the expression of female genes commanding antioxidant enzymes relevant for sperm survival during sperm transport, under predominant estrogen influence through the bloodstream and semen. The findings could contribute to the design of new therapeutics for the female to improve oxidative-reductive balance.
Keywords: ROS; antioxidant; mating; periovulatory; porcine.
Copyright © 2023 Álvarez-Rodríguez, Roca, Martínez and Rodríguez-Martínez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

