Metabolomics reveals the response of hydroprimed maize to mitigate the impact of soil salinization
- PMID: 37351217
- PMCID: PMC10282767
- DOI: 10.3389/fpls.2023.1109460
Metabolomics reveals the response of hydroprimed maize to mitigate the impact of soil salinization
Abstract
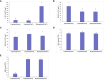
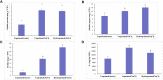
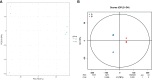
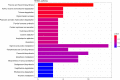
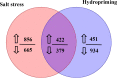
Soil salinization is a major environmental stressor hindering global crop production. Hydropriming has emerged as a promising approach to reduce salt stress and enhance crop yields on salinized land. However, a better mechanisitic understanding is required to improve salt stress tolerance. We used a biochemical and metabolomics approach to study the effect of salt stress of hydroprimed maize to identify the types and variation of differentially accumulated metabolites. Here we show that hydropriming significantly increased catalase (CAT) activity, soluble sugar and proline content, decreased superoxide dismutase (SOD) activity and peroxide (H2O2) content. Conversely, hydropriming had no significant effect on POD activity, soluble protein and MDA content under salt stress. The Metabolite analysis indicated that salt stress significantly increased the content of 1278 metabolites and decreased the content of 1044 metabolites. Ethisterone (progesterone) was the most important metabolite produced in the roots of unprimed samples in response to salt s tress. Pathway enrichment analysis indicated that flavone and flavonol biosynthesis, which relate to scavenging reactive oxygen species (ROS), was the most significant metabolic pathway related to salt stress. Hydropriming significantly increased the content of 873 metabolites and significantly decreased the content of 1313 metabolites. 5-Methyltetrahydrofolate, a methyl donor for methionine, was the most important metabolite produced in the roots of hydroprimed samples in response to salt stress. Plant growth regulator, such as melatonin, gibberellin A8, estrone, abscisic acid and brassinolide involved in both treatment. Our results not only verify the roles of key metabolites in resisting salt stress, but also further evidence that flavone and flavonol biosynthesis and plant growth regulator relate to salt tolerance.
Keywords: hydropriming; land degradation; metabolomics analysis; progesterone; soil salinization.
Copyright © 2023 Zhang, Zhu, Wang, Sun, Tian, Chen, Mou, Zhang, Wei, Fang, Ravenscroft, O’Connor, Chang and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

