Efficacy and safety of Danlou tablets in traditional Chinese medicine for coronary heart disease: a systematic review and meta-analysis
- PMID: 37351285
- PMCID: PMC10282777
- DOI: 10.3389/fcvm.2023.1100006
Efficacy and safety of Danlou tablets in traditional Chinese medicine for coronary heart disease: a systematic review and meta-analysis
Abstract
Background: Danlou tablets exert auxiliary advantages in treating coronary heart disease (CHD), but a summary of evidence-based proof is lacking. This study aims to systematically evaluate Danlou tablets in treating CHD from two aspects, including efficacy and safety.
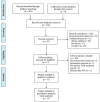
Methods: By a thorough retrieval of the four English databases, namely, PubMed, The Cochrane Library, Embase, and Web of Science, and the four Chinese databases, namely, CNKI, Wanfang, VIP database, and China Biomedical Literature Service System, we found all randomized controlled trials (RCTs) related to Danlou tablets in treating CHD. The retrieval time was from the construction of the database to April 2022. We engaged two researchers to screen the studies, extract the required data, and assess the risk of bias. We then used RevMan5.3 and STATA.14 software to conduct a meta-analysis. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to evaluate the quality of outcome indicators.
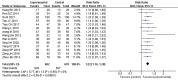
Results: Seventeen RCTs involving 1,588 patients were included. The meta-analysis results are displayed as follows: clinical treatment effect [risk ratio (RR) = 1.22, 95% confidence interval (CI): 1.16, 1.28, P < 0.00001], angina pectoris duration [MD = -0.2.15, 95% CI: -2.91, -1.04, P < 0.00001], angina pectoris frequency [standard mean difference (SMD) = -2.48, 95% CI: -3.42, -1.54, P < 0.00001], angina pectoris degree [SMD = -0.96, 95% CI: -1.39, -0.53, P < 0.0001], TC [MD = -0.71, 95% CI: -0.92, -0.51, P < 0.00001], TG [MD = -0.38, 95% CI: -0.53, -0.22, P < 0.00001], low-density lipoprotein cholesterol [MD = -0.64, 95% CI: -0.76, -0.51, P < 0.00001], high-density lipoprotein cholesterol [MD = 0.16, 95% CI: 0.11, 0.21, P < 0.00001], and adverse events [RR = 0.46, 95% CI: 0.24, 0.88, P = 0.02].
Conclusion: The current evidence suggests that the combination of Danlou tablets and Western medicine can enhance the efficacy of CHD and does not increase adverse events. However, because of the limited number and quality of the included studies, the results of our study should be treated with caution. Further large-scale RCTs are necessary to verify the benefits of this approach.
Keywords: Danlou tablet; complementary alternative therapy; coronary heart disease; meta-analysis; randomized controlled trial.
© 2023 Mao, Lu, Wan, Mao, Lv, Hu, Fu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer Lin Li declared a shared affiliation with the authors RW and YL to the handling editor at the time of review.
Figures






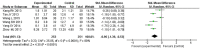
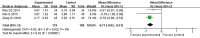
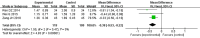
References
-
- Yu YF, Zhou ML, Luo XX, Zhao YC, Zhou XH, Zhang YF, et al. Meta analysis and trial sequential analysis of colchicine in the treatment of coronary heart disease. Chin Circ J. (2021) 36(7):659–66. CNKI:SUN:ZGXH.0.2021-07-006
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

