The safety and efficacy of balloon-expandable versus self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis
- PMID: 37351289
- PMCID: PMC10283153
- DOI: 10.3389/fcvm.2023.1130354
The safety and efficacy of balloon-expandable versus self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis
Erratum in
-
Corrigendum: The safety and efficacy of balloon-expandable versus self-expanding trans-catheter aortic valve replacement in high-risk patients.Front Cardiovasc Med. 2023 Oct 13;10:1282812. doi: 10.3389/fcvm.2023.1282812. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37904807 Free PMC article.
Abstract
Aim: Transfemoral Trans-catheter Aortic Valve Replacement (TF-TAVR) is a safe and effective therapy compared with surgical aortic valve replacement (SAVR) in patients across all risk profiles using balloon-expandable valves (BEV) and self-expanding valves (SEV). Our aim was to compare safety and efficacy of BEV vs. SEV in high-risk patients undergoing TF-TAVR.
Methods and results: We searched PubMed, EMBASE, Clinicaltrials.gov, Scopus, and Web of sciences for studies on patients with severe aortic stenosis undergoing TAVR. Primary outcome was 30-day all-cause mortality. Secondary outcomes defined by Valve Academic Research Consortium 2 (VARC-2) criteria were also examined. Six studies with 2,935 patients (1,439 to BEV and 1,496 to SEV) were included. BEV was associated with lower risk of all-cause mortality (2.2% vs. 4.5%; RR: 0.51; 95% CI: 0.31-0.82; p < 0.006) and cardiovascular mortality [(2.5% vs. 4.3%; RR: 0.54; 95% CI: 0.32-0.90; p = 0.01) at 30 days compared with SEV. Implantation of more than one valve per procedure (0.78% vs. 5.11%; RR: 0.15; 95% CI: 0.07-0.31; p < 0.00001), and moderate/severe AR/PVL (2.5% vs. 9.01%; RR: 0.3; 95% CI: 0.17-0.48); p < 0.00001) were also lower in the BEV arm.
Conclusion: BEV TAVR is associated with reduced all-cause mortality (High level of GRADE evidence), cardiovascular mortality (very low level) at 30 days compared with SEV TAVR in high surgical risk patients. Data are necessary to determine if the difference in outcomes persists in longer-term and if the same effects are seen in lower-risk patients.
Systematic review registration: identifier, CRD42020181190.
Keywords: aortic stenosis; balloon expandable; self-expanding; trans catheter aortic valve replacement; valve.
© 2023 Senguttuvan, Bhatt, Balakrishnan, Krishnamoorthy, Goel, Reddy, Subramanian, Claessen, Kumar, Majmundar, Ro, Lerakis, Jayaraj, Kalra, Flather, Dangas and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
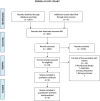


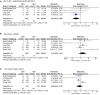


Comment in
-
Commentary: The safety and efficacy of balloon-expandable vs. self-expanding trans-catheter aortic valve replacement in high-risk patients with severe symptomatic aortic stenosis.Front Cardiovasc Med. 2023 Dec 18;10:1295274. doi: 10.3389/fcvm.2023.1295274. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38193022 Free PMC article. No abstract available.
References
-
- Health C for D and R. Edwards SAPIEN 3 transcatheter heart valve system and Edwards SAPIEN 3 ultra transcatheter heart valve system - P140031/S085. FDA (2019) (Accessed April 29, 2020).
-
- Health C for D and R. Medtronic CoreValve system; Medtronic CoreValve Evolut R system; medtronic CoreValve Evolut PRO system - P130021/S033. FDA (2019) (Accessed April 29, 2020).
-
- Health C for D and R. LOTUS Edge™ valve system - P180029. FDA (2019) (Accessed April 29, 2020).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

