A Targeted and pH-Responsive Nano-Graphene Oxide Nanoparticle Loaded with Doxorubicin for Synergetic Chemo-Photothermal Therapy of Oral Squamous Cell Carcinoma
- PMID: 37351329
- PMCID: PMC10284161
- DOI: 10.2147/IJN.S402249
A Targeted and pH-Responsive Nano-Graphene Oxide Nanoparticle Loaded with Doxorubicin for Synergetic Chemo-Photothermal Therapy of Oral Squamous Cell Carcinoma
Abstract
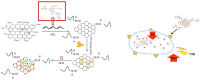
Purpose: Oral squamous cell carcinoma (OSCC) is a malignant disease with serious impacts on human health and quality of life worldwide. This disease is traditionally treated through a combination of surgery, radiotherapy, and chemotherapy. However, the efficacy of traditional treatments is hindered by systemic toxicity, limited therapeutic effects, and drug resistance. Fibroblast activation protein (FAP) is a membrane-bound protease. Although FAP has limited expression in normal adult tissues, it is highly expressed in the tumor microenvironment of many solid cancers - a characteristic that makes it an ideal target for anticancer therapy. In this study, we constructed a nano-drug delivery system (NPF@DOX) targeting FAP to increase the therapeutic efficiency of synergistic chemo-photothermal therapy against OSCC.
Methods: We utilized PEGylated nano-graphene oxide (NGO) to link doxorubicin (DOX) and fluorescently-labeled, FAP-targeted peptide chains via hydrogen bonding and π-π bonding to enhance the targeting capability of NPF@DOX. The synthesis of NPF@DOX was analyzed using UV-Vis and FT-IR spectroscopy and its morphology using transmission electron microscopy (TEM). Additionally, the drug uptake efficiency in vitro, photo-thermal properties, release performance, and anti-tumor effects of NPF@DOX were evaluated and further demonstrated in vivo.
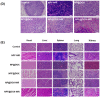
Results: Data derived from FT-IR, UV-Vis, and TEM implied successful construction of the NPF@DOX nano-drug delivery system. Confocal laser scanning microscopy images and in vivo experiments demonstrated the targeting effects of FAP on OSCC. Furthermore, NPF@DOX exhibited a high photothermal conversion efficiency (52.48%) under near-infrared radiation. The thermogenic effect of NPF@DOX simultaneously promoted local release of DOX and apoptosis based on a pH-stimulated effect. Importantly, FAP-targeted NPF@DOX in combination with PTT showed better tumor suppression performance in vivo and in vitro than did either therapy individually.
Conclusion: NPF@DOX can precisely target OSCC, and combined treatment with chemical and photothermal therapy can improve the therapeutic outcomes of OSCC. This method serves as an efficient therapeutic strategy for the development of synergistic anti-tumor research.
Keywords: fibroblast activation protein; nano-graphene oxide; oral squamous cell carcinoma; photothermal therapy; targeted combination therapy.
© 2023 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

