Clinical validation of 3D-printed swabs in adults and children for SARS-CoV-2 detection
- PMID: 37351376
- PMCID: PMC10281960
- DOI: 10.1093/biomethods/bpad009
Clinical validation of 3D-printed swabs in adults and children for SARS-CoV-2 detection
Abstract
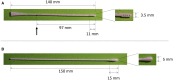
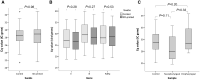
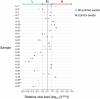
Throughout the entire coronavirus disease 19 (COVID-19) pandemic, there were disruptions in the supply chain of test materials around the world, primarily in poor- and middle-income countries. The use of 3D prints is an alternative to address swab supply shortages. In this study, the feasibility of the clinical use of 3D-printed swabs for oropharyngeal and nasopharyngeal sampling for the detection of SARS-CoV-2 infection was evaluated. For that purpose, paired samples with the 3D printed and the control swabs were taken from 42 adult patients and 10 pediatric patients, and the results obtained in the detection of SARS-CoV-2 by reverse transcription and quantitative polymerase chain reaction (RT-qPCR) were compared. Additionally, in those cases where the result was positive for SARS-CoV-2, the viral load was calculated by means of a mathematical algorithm proposed by us. For both adults and children, satisfactory results were obtained in the detection of SARS-CoV-2 by RT-qPCR; no significant differences were found in the quantification cycle values between the 3D-printed swab samples and the control samples. Furthermore, we corroborated that the 3D-printed swabs caused less discomfort and pain at the time of sampling. In conclusion, this study shows the feasibility of routinely using 3D-printed swabs for both adults and children. In this way, it is possible to maintain local and cheaper consumption along with fewer distribution difficulties.
Keywords: 3D-printed swabs; COVID-19; SARS-CoV-2; clinical validation; polylactic acid; thermoplastic elastomers.
© The Author(s) 2023. Published by Oxford University Press.
Figures
Similar articles
-
Validation of 3D-Printed Swabs for Sampling in SARS-CoV-2 Detection: A Pilot Study.Ann Biomed Eng. 2023 Mar;51(3):527-537. doi: 10.1007/s10439-022-03057-1. Epub 2022 Sep 12. Ann Biomed Eng. 2023. PMID: 36094762 Free PMC article.
-
Pandemic printing: a novel 3D-printed swab for detecting SARS-CoV-2.Med J Aust. 2020 Sep;213(6):276-279. doi: 10.5694/mja2.50726. Epub 2020 Aug 9. Med J Aust. 2020. PMID: 32772375 Free PMC article.
-
3-Dimensional Printed Alternative to the Standard Synthetic Flocked Nasopharyngeal Swabs Used for Coronavirus Disease 2019 Testing.Clin Infect Dis. 2021 Nov 2;73(9):e3027-e3032. doi: 10.1093/cid/ciaa1366. Clin Infect Dis. 2021. PMID: 32910817 Free PMC article. Clinical Trial.
-
Design and Multicenter Clinical Validation of a 3-Dimensionally Printed Nasopharyngeal Swab for SARS-CoV-2 Testing.JAMA Otolaryngol Head Neck Surg. 2021 May 1;147(5):418-425. doi: 10.1001/jamaoto.2020.5680. JAMA Otolaryngol Head Neck Surg. 2021. PMID: 33599684 Free PMC article.
-
Low-Cost Polyester-Tipped Three-Dimensionally Printed Nasopharyngeal Swab for the Detection of Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2).J Clin Microbiol. 2020 Oct 21;58(11):e01668-20. doi: 10.1128/JCM.01668-20. Print 2020 Oct 21. J Clin Microbiol. 2020. PMID: 32817232 Free PMC article.
References
-
- Ochani R, Asad A, Yasmin F et al. COVID-19 pandemic: From origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med 2021;29:20–36. - PubMed
-
- Das K, Pingali MS, Paital B et al. A detailed review of the outbreak of COVID-19. Front Biosci (Landmark Ed) 2021;26:149–70. - PubMed
-
- WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (11 April 2023, date last accessed).
LinkOut - more resources
Full Text Sources
Miscellaneous
