Efficacy and Safety of Adintrevimab (ADG20) for the Treatment of High-Risk Ambulatory Patients With Mild or Moderate Coronavirus Disease 2019: Results From a Phase 2/3, Randomized, Placebo-Controlled Trial (STAMP) Conducted During Delta Predominance and Early Emergence of Omicron
- PMID: 37351456
- PMCID: PMC10284338
- DOI: 10.1093/ofid/ofad279
Efficacy and Safety of Adintrevimab (ADG20) for the Treatment of High-Risk Ambulatory Patients With Mild or Moderate Coronavirus Disease 2019: Results From a Phase 2/3, Randomized, Placebo-Controlled Trial (STAMP) Conducted During Delta Predominance and Early Emergence of Omicron
Abstract
Background: Safe and effective treatments are needed to prevent severe outcomes in individuals with coronavirus disease 2019 (COVID-19). We report results from STAMP, a phase 2/3, multicenter, double-blind, randomized, placebo-controlled trial of adintrevimab, an extended half-life monoclonal antibody, for treatment of high-risk ambulatory patients with mild to moderate COVID-19.
Methods: Nonhospitalized, unvaccinated participants aged ≥12 years with mild to moderate COVID-19 and ≥1 risk factor for disease progression were randomized to receive a single intramuscular injection of 300 mg adintrevimab or placebo. Enrollment was paused due to the global emergence of the Omicron BA.1/BA1.1 variants, against which adintrevimab showed reduced activity in vitro. The primary efficacy endpoint was COVID-19-related hospitalization or all-cause death through day 29 in participants with COVID-19 due to laboratory-confirmed or suspected non-Omicron severe acute respiratory syndrome coronavirus 2 variants.
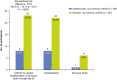
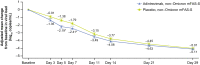
Results: Between 8 August 2021 and 11 January 2022, 399 participants were randomized to receive adintrevimab (n = 198) or placebo (n = 201), including 336 with COVID-19 due to non-Omicron variants. COVID-19-related hospitalization or all-cause death through day 29 occurred in 8 of 169 (4.7%) participants in the adintrevimab group and 23 of 167 (13.8%) participants in the placebo group, a 66% relative risk reduction in favor of adintrevimab (standardized risk difference, -8.7% [95% confidence interval, -14.71% to -2.67%]; P = .0047). Incidence of treatment-emergent adverse events (TEAEs) was similar between treatment groups (33.9% for adintrevimab and 39.5% for placebo). No adintrevimab-related serious TEAEs were reported.
Conclusions: Treatment with a single intramuscular injection of adintrevimab provided protection against severe outcomes in high-risk ambulatory participants with COVID-19 due to susceptible variants, without safety concerns. Clinical Trial Registration. NCT04805671.
Keywords: COVID-19; SARS-CoV-2; adintrevimab; monoclonal antibody; treatment.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. G. I. has received research support, paid to Northwestern University, from GlaxoSmithKline; payment for consultation from ADMA Biologics, AlloVir, Atea, Cidara, Genentech, Invivyd, Roche, Janssen, Shionogi, Takeda, and Viracor Eurofins; royalties from UpToDate; and payment for serving on independent data monitoring committees for Adamis, AlloVir, CSL Seqirus, Merck, Takeda, and Talaris. M. P., K. M., N. B., Y. L., D. G., K. N., E. H., L. E. C., I. Y., M. S., E. C., P. H., and P. S. were employees of Invivyd at the time the study was conducted, and may own stock or shares of Invivyd, Inc. A. F. D. and J. G. were paid consultants to Invivyd at the time the study was conducted but did not receive any financial renumeration for work done reviewing the manuscript. L. E. C. was a paid consultant to Invivyd at the time the manuscript was prepared but did not receive any financial renumeration for work done reviewing the manuscript. As study investigators, N. E. and M. T. were compensated by Invivyd for all patient visits including enrollment/baseline and follow-up.
Figures

References
-
- Coronavirus Resource Center . Global deaths. Available at: https://coronavirus.jhu.edu. Accessed 17 January 2023.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

