Adjustment of creatinine clearance for carboplatin dosing in Calvert's formula and clinical efficacy for lung cancer
- PMID: 37351560
- PMCID: PMC10469651
- DOI: 10.1002/cam4.6235
Adjustment of creatinine clearance for carboplatin dosing in Calvert's formula and clinical efficacy for lung cancer
Abstract
Background: The Cockcroft-Gault formula is commonly used as a substitute for glomerular filtration rate (GFR) in Calvert's formula for carboplatin dosing, where adjusting serum creatinine measured using the enzymatic method with 0.2 mg/dL has been suggested in Japan. However, the effects of these adjustments on efficacy in patients with non-small-cell lung cancer remain unknown.
Methods: We conducted a post hoc analysis of the PREDICT1 study (CJLSG1201), a multicenter prospective observational trial of carboplatin-pemetrexed. Glomerular filtration rate values in Calvert's formula were back-calculated from the administered dosages of carboplatin and the reported value of the target area under the curve. We estimated the serum creatinine adjustments and divided the patients into crude and adjusted groups.
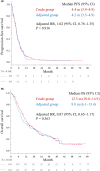
Results: Patients in the crude group (N = 169) demonstrated similar efficacy to those in the adjusted group (N = 104) in progression-free survival (PFS) and overall survival (OS) (hazard ratio [HR], 1.02; 95% confidence interval [CI], 0.76-1.35; p = 0.916 vs. HR, 0.87; 95% CI, 0.65-1.17; p = 0.363), with higher grade 3-4 hematologic toxicity. Among patients aged ≥75 years, the crude group (N = 47) showed superior efficacy compared with the adjusted group (N = 17) in PFS and OS (HR, 0.37; 95% CI, 0.20-0.69; p = 0.002 vs. HR, 0.43; 95% CI, 0.23-0.82; p = 0.010).
Conclusions: Serum creatinine adjustment may be associated with similar efficacy compared to the crude serum creatinine value. In older patients, the adjustment should be cautiously applied owing to the potential for reduced efficacy.
Keywords: Cockcroft-Gault formula; carboplatin; creatinine clearance; glomerular filtration rate; non-small-cell lung cancer.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
T. Hase received research funding from AstraZeneca, Chugai Pharmaceutical Co., Ltd., and Novartis Pharma. K.K. Y. Ando received personal fees from Chugai Pharmaceutical Co. and research funding from Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Chugai Pharmaceutical Co., Ltd., BeiGene Inc., and Geo Holdings Co., Ltd. Y. Hasegawa received personal fees from Novartis Pharma K.K., Boehringer Ingelheim, and AstraZeneca. The grant was paid to each institution. All remaining authors have no conflicts of interest to declare.
Figures

References
-
- Harland SJ, Newell DR, Siddik ZH, Chadwick R, Calvert AH, Harrap KR. Pharmacokinetics of cis‐diammine‐1,1‐cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984;44(4):1693‐1697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

