Association between vaccination and the risk of central demyelination: results from a case-referent study
- PMID: 37351662
- PMCID: PMC10511379
- DOI: 10.1007/s00415-023-11822-y
Association between vaccination and the risk of central demyelination: results from a case-referent study
Abstract
Background: Few studies documented the potential association between vaccination and the risk of central demyelination (CD). Specifically, anti-hepatitis B and anti-human papillomavirus (HPV) vaccines have been the subject of distrust with regard to their implication to trigger CD.
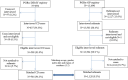
Methods: From a systematic national registry, patients with first signs of CD (cases) were identified and documented for their exposure to vaccination up to 24 months before the first signs occurred. This exposure was compared to that of a representative sample of general practice patients without a history of CD, randomly selected from a national registry (referents). CD cases were 2:1 matched on age, sex, index date (ID), and region of residence. Vaccines against influenza, HPV, hepatitis B and diphtheria-tetanus-pertussis-poliomyelitis-haemophilus (DTPPHae) were considered. Associations between vaccination and CD were assessed using multivariate conditional logistic regressions, controlled for confounding factors.
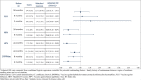
Findings: 564 CD cases were matched to 1,128 randomly selected referents (age range: 2-79 years old). Overall, 123 (22%) CD cases and 320 (28%) referents had received at least one vaccine within 24 months before ID. Adjusted odds ratios (ORs) for any vaccination were 0.69, 95% confidence interval (CI) [0.54-0.88] with respect to any CD first signs, 0.68 [0.51-0.90] for myelitis and 0.70 [0.42-1.17] for optic neuritis. Adjusted ORs for any CD first signs were 1.02 [0.71-1.47] for influenza vaccine (administered in 9.6% of cases and 10.4% of referents) and 0.72 [0.53-0.99] for DTPPHae vaccine (administered in 10.8% of cases and 14.5% of referents). Vaccines against hepatitis B and HPV were only administered in 1.1% and 1.2% of cases and in 2.9% and 3.2% of referents respectively, which statistically explained the point estimates < 1 (ORs of 0.39 [0.16-0.94] and of 0.32 [0.13-0.80]).
Interpretation: No increased risk of CD incidence was observed amongst vaccinated patients. Lower rates of vaccination against hepatitis B and HPV observed in patients with CD compared to referents may be due to the reluctance of physicians to vaccinate patients considered at risk of CD.
Keywords: Case-referent study; Central demyelination; Multiple sclerosis; Vaccines.
© 2023. The Author(s).
Conflict of interest statement
LG-B was the recipient of a research fellowship from INSERM (French National Institute of Health and Medical Research) at the time of the study and was employed by LA-SER, the company conducting the study. CP is a member of the board for Bayer Schering Pharma, Novartis, Roche, Teva Pharmaceutical Industries Ltd and Sanofi-Genzyme Bio Ventures; she has also received payment for lectures and participation in speakers’ bureaus from Bayer Schering Pharma, Novartis, Teva Pharmaceutical Industries Ltd, Merck Serono SA, Biogen Idec and Genzyme. YH was employed by LA-SER, the company conducting the study. JB was a consultant for LA-SER. LA was a stock-holder and chairman of LA-SER and Centre for Risk Research Inc., an independent research organisation that owns and develops the PGRx database. LA-SER and Centre for Risk Research Inc. have no commercial interests in any of the products investigated in this study.
Figures
Similar articles
-
Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus.Pediatrics. 2001 Dec;108(6):E112. doi: 10.1542/peds.108.6.e112. Pediatrics. 2001. PMID: 11731639
-
Risk of autoimmune diseases and human papilloma virus (HPV) vaccines: Six years of case-referent surveillance.J Autoimmun. 2017 May;79:84-90. doi: 10.1016/j.jaut.2017.01.005. Epub 2017 Feb 9. J Autoimmun. 2017. PMID: 28190705
-
Vaccinations and risk of central nervous system demyelinating diseases in adults.Arch Neurol. 2003 Apr;60(4):504-9. doi: 10.1001/archneur.60.4.504. Arch Neurol. 2003. PMID: 12707063
-
Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa).BioDrugs. 2010 Oct 1;24(5):299-302. doi: 10.2165/11206690-000000000-00000. BioDrugs. 2010. PMID: 20795752 Review.
-
Integration of hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliomyelitis and Haemophilus influenzae type b conjugate vaccine within existing national recommendations following a birth dose of monovalent hepatitis B virus vaccine: results of a systematic review in the Asia Pacific region.Expert Rev Vaccines. 2019 Sep;18(9):921-933. doi: 10.1080/14760584.2019.1646643. Epub 2019 Aug 1. Expert Rev Vaccines. 2019. PMID: 31328999
Cited by
-
Environmental risk factors for multiple sclerosis: a comprehensive systematic review and meta-analysis.J Neurol. 2025 Jul 15;272(8):513. doi: 10.1007/s00415-025-13248-0. J Neurol. 2025. PMID: 40664781 Free PMC article. Review.
-
Case report: Satralizumab therapy for bilateral refractory optic neuritis following the first dose of bivalent human papilloma virus vaccine.Front Immunol. 2024 Nov 19;15:1499045. doi: 10.3389/fimmu.2024.1499045. eCollection 2024. Front Immunol. 2024. PMID: 39628490 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical