Prenatal diagnosis of bone dysplasias
- PMID: 37351952
- PMCID: PMC10321247
- DOI: 10.1259/bjr.20221025
Prenatal diagnosis of bone dysplasias
Abstract
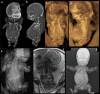
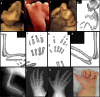
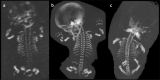
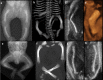
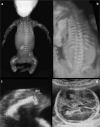
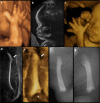
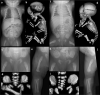
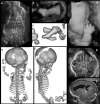
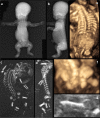
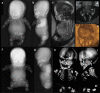
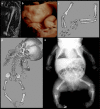
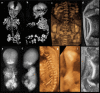
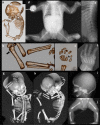
Bone dysplasias are individually rare but collectively common. The prenatal diagnosis of bone dysplasias, especially perinatally lethal dysplasias, is of major interest to obstetric services. The current nosology of genetic skeletal disorders addresses over 400 disorders. However, in clinical practice, we encounter only a limited number of disorders, such as FGFR3-related dysplasias, osteogenesis imperfecta, and type II collagenopathies. The recent development of non-invasive prenatal genetic testing using cell-free fetal DNA in maternal blood samples has had a major impact on the prenatal diagnosis of genetic diseases. However, imaging examinations remain critical for the final diagnosis of bone dysplasias because molecular testing only shows genetic variants, and not their pathogenicity - most variants are clinically insignificant. Bone dysplasias are typically suspected when limb shortening is identified by screening ultrasound. Further assessment can be followed by more detailed ultrasound, magnetic resonance imaging (MRI), and CT. Based on these data, rational decision-making is feasible, even when the definitive prenatal diagnosis is not feasible. Here, we highlight key images of common bone dysplasias obtained by currently available modalities.
Figures


References
-
- Connor JM, Connor RA, Sweet EM, Gibson AA, Patrick WJ, McNay MB, et al. . Lethal neonatal chondrodysplasias in the West of Scotland 1970-1983 with a description of a thanatophoric, dysplasialike, autosomal recessive disorder, Glasgow variant. Am J Med Genet 1985; 22: 243–53. doi: 10.1002/ajmg.1320220205 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

