Identification of a biosynthetic gene cluster for a red pigment cristazarin produced by a lichen-forming fungus Cladonia metacorallifera
- PMID: 37352186
- PMCID: PMC10289310
- DOI: 10.1371/journal.pone.0287559
Identification of a biosynthetic gene cluster for a red pigment cristazarin produced by a lichen-forming fungus Cladonia metacorallifera
Abstract
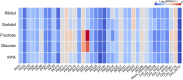
Lichens are known to produce many novel bioactive metabolites. To date, approximately 1,000 secondary metabolites have been discovered, which are predominantly produced by the lichen mycobionts. However, despite the extensive studies on production of lichen secondary metabolites, little is known about the responsible biosynthetic gene clusters (BGCs). Here, we identified a putative BGC that is implicated in production of a red pigment, cristazarin (a naphthazarin derivative), in Cladonia metacorallifera. Previously, cristazarin was shown to be specifically induced in growth media containing fructose as a sole carbon source. Thus, we performed transcriptome analysis of C. metacorallifera growing on different carbon sources including fructose to identify the BGC for cristazarin. Among 39 polyketide synthase (PKS) genes found in the genome of C. metacorallifera, a non-reducing PKS (coined crz7) was highly expressed in growth media containing either fructose or glucose. The borders of a cristazarin gene cluster were delimited by co-expression patterns of neighboring genes of the crz7. BGCs highly conserved to the cristazarin BGC were also found in C. borealis and C. macilenta, indicating that these related species also have metabolic potentials to produce cristazarin. Phylogenetic analysis revealed that the Crz7 is sister to fungal PKSs that biosynthesize an acetylated tetrahydoxynaphthalene as a precursor of melanin pigment. Based on the phylogenetic placement of the Crz7 and putative functions of its neighboring genes, we proposed a plausible biosynthetic route for cristazarin. In this study, we identified a lichen-specific BGC that is likely involved in the biosynthesis of a naphthazarin derivative, cristazarin, and confirmed that transcriptome profiling under inducing and non-inducing conditions is an effective strategy for linking metabolites of interest to biosynthetic genes.
Copyright: © 2023 Paguirigan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Boustie J, Grube M. Lichens—a promising source of bioactive secondary metabolites. Plant Gen Resourc 2005;3(2):273–287.
-
- Goga M, Elečko J, Marcinčinová M, Ručová D, Bačkorová M, Backor M. Lichen metabolites: an overview of some secondary metabolites and their biological potential. In co-evolution of secondary metabolites, reference series in phytochemistry, eds J.-M. Mérillon and K. G. Ramawat (Berlin: Springer Nature). 2018. p. 1–36.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

