Effects of elastase-induced emphysema on muscle and bone in mice
- PMID: 37352205
- PMCID: PMC10289373
- DOI: 10.1371/journal.pone.0287541
Effects of elastase-induced emphysema on muscle and bone in mice
Abstract
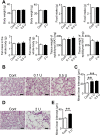
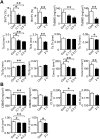
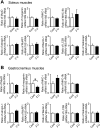
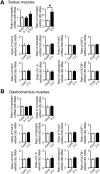
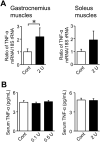
Chronic obstructive pulmonary disease (COPD) causes sarcopenia and osteoporosis. However, the mechanisms underlying muscle and bone loss as well as the interactions between muscle and bone in the COPD state remain unclear. Therefore, we herein investigated the effects of the COPD state on muscle and bone in mice intratracheally administered porcine pancreatic elastase (PPE). The intratracheal administration of PPE to mice significantly reduced trabecular bone mineral density (BMD), trabecular bone volume, trabecular number, cortical BMD and cortical area. It also significantly decreased grip strength, but did not affect muscle mass or the expression of myogenic differentiation-, protein degradation- or autophagy-related genes in the soleus and gastrocnemius muscles. Among the myokines examined, myostatin mRNA levels in the soleus muscles were significantly elevated in mice treated with PPE, and negatively related to grip strength, but not bone parameters, in mice treated with or without 2 U PPE in simple regression analyses. Grip strength positively related to bone parameters in mice treated with or without PPE. In conclusion, we showed that a PPE model of COPD in mice exerts dominant effects on bone rather than skeletal muscles. Increased myostatin expression in the soleus muscles of mice in the COPD state may negatively relate to a reduction in grip strength, but not bone loss.
Copyright: © 2023 Matsumura et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

