Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice
- PMID: 37352350
- PMCID: PMC10289645
- DOI: 10.1126/sciadv.adf4683
Optogenetic-induced muscle loading leads to mechanical adaptation of the Achilles tendon enthesis in mice
Abstract
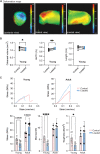
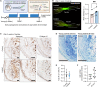
Skeletal shape depends on the transmission of contractile muscle forces from tendon to bone across the enthesis. Loss of muscle loading impairs enthesis development, yet little is known if and how the postnatal enthesis adapts to increased loading. Here, we studied adaptations in enthesis structure and function in response to increased loading, using optogenetically induced muscle contraction in young (i.e., growth) and adult (i.e., mature) mice. Daily bouts of unilateral optogenetic loading in young mice led to radial calcaneal expansion and warping. This also led to a weaker enthesis with increased collagen damage in young tendon and enthisis, with little change in adult mice. We then used RNA sequencing to identify the pathways associated with increased mechanical loading during growth. In tendon, we found enrichment of glycolysis, focal adhesion, and cell-matrix interactions. In bone, we found enrichment of inflammation and cell cycle. Together, we demonstrate the utility of optogenetic-induced muscle contraction to elicit in vivo adaptation of the enthesis.
Figures






Update of
-
Optogenetic-Induced Muscle Loading Leads to Mechanical Adaptation of the Achilles Tendon Enthesis in Mice.bioRxiv [Preprint]. 2023 May 2:2023.04.11.536376. doi: 10.1101/2023.04.11.536376. bioRxiv. 2023. Update in: Sci Adv. 2023 Jun 23;9(25):eadf4683. doi: 10.1126/sciadv.adf4683. PMID: 37090593 Free PMC article. Updated. Preprint.
References
-
- L. Rossetti, L. A. Kuntz, E. Kunold, J. Schock, K. W. Müller, H. Grabmayr, J. Stolberg-Stolberg, F. Pfeiffer, S. A. Sieber, R. Burgkart, A. R. Bausch, The microstructure and micromechanics of the tendon–bone insertion. Nat. Mater. 16, 664–670 (2017). - PubMed
-
- M. Benjamin, D. McGonagle, "The enthesis organ concept and its relevance to the spondyloarthropathies" in Molecular Mechanisms of Spondyloarthropathies, C. López-Larrea, R. Díaz-Peña, Eds. (Springer, 2009), pp. 57–70. - PubMed
-
- S. Thomopoulos, J. P. Marquez, B. Weinberger, V. Birman, G. M. Genin, Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech. 39, 1842–1851 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

