Unique amphipathic α helix drives membrane insertion and enzymatic activity of ATG3
- PMID: 37352354
- PMCID: PMC10289646
- DOI: 10.1126/sciadv.adh1281
Unique amphipathic α helix drives membrane insertion and enzymatic activity of ATG3
Abstract
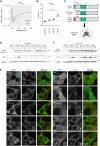
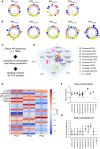
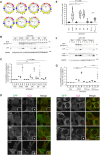
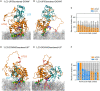
Autophagosome biogenesis requires a localized perturbation of lipid membrane dynamics and a unique protein-lipid conjugate. Autophagy-related (ATG) proteins catalyze this biogenesis on cellular membranes, but the underlying molecular mechanism remains unclear. Focusing on the final step of the protein-lipid conjugation reaction, the ATG8/LC3 lipidation, we show how the membrane association of the conjugation machinery is organized and fine-tuned at the atomistic level. Amphipathic α helices in ATG3 proteins (AHATG3) have low hydrophobicity and contain less bulky residues. Molecular dynamics simulations reveal that AHATG3 regulates the dynamics and accessibility of the thioester bond of the ATG3~LC3 conjugate to lipids, enabling the covalent lipidation of LC3. Live-cell imaging shows that the transient membrane association of ATG3 with autophagic membranes is governed by the less bulky-hydrophobic feature of AHATG3. The unique properties of AHATG3 facilitate protein-lipid bilayer association, leading to the remodeling of the lipid bilayer required for the formation of autophagosomes.
Figures


References
-
- Mizushima N., Yoshimori T., Ohsumi Y., The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011). - PubMed
-
- Nakatogawa H., Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 21, 439–458 (2020). - PubMed
-
- van Vliet A. R., Chiduza G. N., Maslen S. L., Pye V. E., Joshi D., De Tito S., Jefferies H. B. J., Christodoulou E., Roustan C., Punch E., Hervás J. H., O'Reilly N., Skehel J. M., Cherepanov P., Tooze S. A., ATG9A and ATG2A form a heteromeric complex essential for autophagosome formation. Mol. Cell 82, 4324–4339.e8 (2022). - PubMed
-
- Noda N. N., Atg2 and Atg9: Intermembrane and interleaflet lipid transporters driving autophagy. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 158956 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

