Genetic regulators of sputum mucin concentration and their associations with COPD phenotypes
- PMID: 37352370
- PMCID: PMC10325042
- DOI: 10.1371/journal.pgen.1010445
Genetic regulators of sputum mucin concentration and their associations with COPD phenotypes
Abstract
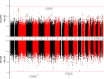
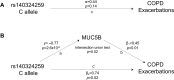
Hyper-secretion and/or hyper-concentration of mucus is a defining feature of multiple obstructive lung diseases, including chronic obstructive pulmonary disease (COPD). Mucus itself is composed of a mixture of water, ions, salt and proteins, of which the gel-forming mucins, MUC5AC and MUC5B, are the most abundant. Recent studies have linked the concentrations of these proteins in sputum to COPD phenotypes, including chronic bronchitis (CB) and acute exacerbations (AE). We sought to determine whether common genetic variants influence sputum mucin concentrations and whether these variants are also associated with COPD phenotypes, specifically CB and AE. We performed a GWAS to identify quantitative trait loci for sputum mucin protein concentration (pQTL) in the Sub-Populations and InteRmediate Outcome Measures in COPD Study (SPIROMICS, n = 708 for total mucin, n = 215 for MUC5AC, MUC5B). Subsequently, we tested for associations of mucin pQTL with CB and AE using regression modeling (n = 822-1300). Replication analysis was conducted using data from COPDGene (n = 5740) and by examining results from the UK Biobank. We identified one genome-wide significant pQTL for MUC5AC (rs75401036) and two for MUC5B (rs140324259, rs10001928). The strongest association for MUC5B, with rs140324259 on chromosome 11, explained 14% of variation in sputum MUC5B. Despite being associated with lower MUC5B, the C allele of rs140324259 conferred increased risk of CB (odds ratio (OR) = 1.42; 95% confidence interval (CI): 1.10-1.80) as well as AE ascertained over three years of follow up (OR = 1.41; 95% CI: 1.02-1.94). Associations between rs140324259 and CB or AE did not replicate in COPDGene. However, in the UK Biobank, rs140324259 was associated with phenotypes that define CB, namely chronic mucus production and cough, again with the C allele conferring increased risk. We conclude that sputum MUC5AC and MUC5B concentrations are associated with common genetic variants, and the top locus for MUC5B may influence COPD phenotypes, in particular CB.
Copyright: © 2023 Buren et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: EKS received grant support from GlaxoSmithKline and Bayer. ESW received honoraria from Pri-Med. JAK reports a grant from U.S. National Institutes of Health (NIH) paid to the institution during the conduct of the study. He also reports personal fees from GlaxoSmithKline consulting on antibodies for acute COVID-19; personal fees from AstraZeneca consulting on antibodies for severe asthma, paid to the institution; personal fees from CereVu Medical consultant for medical device for dyspnea, paid to the institution; and personal fees from BData consultant for severe asthma registry outside the submitted work. JLC reports grants from NIH/NHLBI and the COPD Foundation for the current work and paid to his institution; grants from NIH/NHLBI, the COPD Foundation, NIH/NIAID, and the Departments of Veterans Affairs and of Defense, outside the current work and paid to his institution; and consulting fees from CSL Behring, LLC, AstraZenca, and Novartis Corp., outside the current work and paid to his institution. MCN has received grant support from GlaxoSmithKline. MHC has received grant support from GlaxoSmithKline and Bayer, consulting fees from AstraZeneca, and speaking fees from Illumina. MK received consulting fees from Arrowhead pharmaceuticals and has a patent pending for mucin measurements in chronic bronchitis. MKH reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Amgen, UpToDate, Altesa Biopharma, Medscape, NACE, MDBriefcase, Integrity and Medwiz. She has received either in kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, Astrazeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines and Altesa Biopharma. MVDB reports research grants paid to their institution by GlaxoSmithKline, Novartis, AstraZeneca, Roche and Genentech. SAC received consulting fees from Sanofi/Regeneron, GlaxoSmithKline, AstraZeneca, Glenmark Pharmaceuticals, and Amgen; honoraria from Sanofi/Regeneron, MJH Holdings LLC: Physicians? Education Resource, Sunovion, UpToDate, and Wolters Kluwer Health; travel support from AstraZeneca and GlaxoSmithKline; and sits on the data safety board or advisory boards of Sanofi/Regeneron, and AstraZeneca, and GlaxoSmithKline. TL has stock options from Variant Bio. VEO is on the Data Safety Monitoring Board or Advisory Board for Sanofi and Regeneron. ATH, EVB, GR, HD, SG, SL, SK, SNPK, WKO, and YL have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- HHSN268201800001C/HL/NHLBI NIH HHS/United States
- R01 HL142028/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- U01 DA052713/DA/NIDA NIH HHS/United States
- HHSN268201500014C/HL/NHLBI NIH HHS/United States
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- U01 HL120393/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- R01 HL122711/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- R01 HL110906/HL/NHLBI NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- R01 HL117626/HL/NHLBI NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

