Rewilding of laboratory mice enhances granulopoiesis and immunity through intestinal fungal colonization
- PMID: 37352372
- PMCID: PMC10350741
- DOI: 10.1126/sciimmunol.add6910
Rewilding of laboratory mice enhances granulopoiesis and immunity through intestinal fungal colonization
Abstract
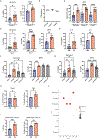
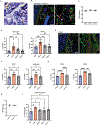
The paucity of blood granulocyte populations such as neutrophils in laboratory mice is a notable difference between this model organism and humans, but the cause of this species-specific difference is unclear. We previously demonstrated that laboratory mice released into a seminatural environment, referred to as rewilding, display an increase in blood granulocytes that is associated with expansion of fungi in the gut microbiota. Here, we find that tonic signals from fungal colonization induce sustained granulopoiesis through a mechanism distinct from emergency granulopoiesis, leading to a prolonged expansion of circulating neutrophils that promotes immunity. Fungal colonization after either rewilding or oral inoculation of laboratory mice with Candida albicans induced persistent expansion of myeloid progenitors in the bone marrow. This increase in granulopoiesis conferred greater long-term protection from bloodstream infection by gram-positive bacteria than by the trained immune response evoked by transient exposure to the fungal cell wall component β-glucan. Consequently, introducing fungi into laboratory mice may restore aspects of leukocyte development and provide a better model for humans and free-living mammals that are constantly exposed to environmental fungi.
Figures





References
-
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R, Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004). - PubMed
-
- Smith K, McCoy KD, Macpherson AJ, Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol 19, 59–69 (2007). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

