Null and missense mutations of ERI1 cause a recessive phenotypic dichotomy in humans
- PMID: 37352860
- PMCID: PMC10357479
- DOI: 10.1016/j.ajhg.2023.06.001
Null and missense mutations of ERI1 cause a recessive phenotypic dichotomy in humans
Abstract
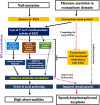
ERI1 is a 3'-to-5' exoribonuclease involved in RNA metabolic pathways including 5.8S rRNA processing and turnover of histone mRNAs. Its biological and medical significance remain unclear. Here, we uncover a phenotypic dichotomy associated with bi-allelic ERI1 variants by reporting eight affected individuals from seven unrelated families. A severe spondyloepimetaphyseal dysplasia (SEMD) was identified in five affected individuals with missense variants but not in those with bi-allelic null variants, who showed mild intellectual disability and digital anomalies. The ERI1 missense variants cause a loss of the exoribonuclease activity, leading to defective trimming of the 5.8S rRNA 3' end and a decreased degradation of replication-dependent histone mRNAs. Affected-individual-derived induced pluripotent stem cells (iPSCs) showed impaired in vitro chondrogenesis with downregulation of genes regulating skeletal patterning. Our study establishes an entity previously unreported in OMIM and provides a model showing a more severe effect of missense alleles than null alleles within recessive genotypes, suggesting a key role of ERI1-mediated RNA metabolism in human skeletal patterning and chondrogenesis.
Keywords: ERI1; exoribonuclease; ribosomopathy; short stature; skeletal dysplasia; spondyloepimetaphyseal dysplasia.
Copyright © 2023 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics Laboratories.
Figures






References
-
- Simmer F., Tijsterman M., Parrish S., Koushika S.P., Nonet M.L., Fire A., Ahringer J., Plasterk R.H.A. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. Elegans hypersensitive to RNAi. Curr. Biol. 2002;12:1317–1319. - PubMed
-
- Bühler M., Verdel A., Moazed D. Tethering RITS to a Nascent Transcript Initiates RNAi- and Heterochromatin-Dependent Gene Silencing. Cell. 2006;125:873–886. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

