Pushing the boundaries of brain organoids to study Alzheimer's disease
- PMID: 37353408
- PMCID: PMC10374393
- DOI: 10.1016/j.molmed.2023.05.007
Pushing the boundaries of brain organoids to study Alzheimer's disease
Abstract
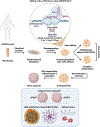
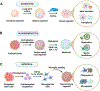
Progression of Alzheimer's disease (AD) entails deterioration or aberrant function of multiple brain cell types, eventually leading to neurodegeneration and cognitive decline. Defining how complex cell-cell interactions become dysregulated in AD requires novel human cell-based in vitro platforms that could recapitulate the intricate cytoarchitecture and cell diversity of the human brain. Brain organoids (BOs) are 3D self-organizing tissues that partially resemble the human brain architecture and can recapitulate AD-relevant pathology. In this review, we highlight the versatile applications of different types of BOs to model AD pathogenesis, including amyloid-β and tau aggregation, neuroinflammation, myelin breakdown, vascular dysfunction, and other phenotypes, as well as to accelerate therapeutic development for AD.
Keywords: Alzheimer’s disease; Apolipoprotein E4; brain organoids; drug discovery; induced pluripotent stem cells; myelin organoids.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests G.B. is an employee of SciNeuro Pharmaceuticals. The remaining authors have no interests to declare.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

