Histone demethylases in the regulation of immunity and inflammation
- PMID: 37353521
- PMCID: PMC10290154
- DOI: 10.1038/s41420-023-01489-9
Histone demethylases in the regulation of immunity and inflammation
Abstract
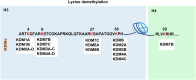
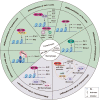
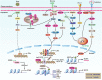
Pathogens or danger signals trigger the immune response. Moderate immune response activation removes pathogens and avoids excessive inflammation and tissue damage. Histone demethylases (KDMs) regulate gene expression and play essential roles in numerous physiological processes by removing methyl groups from lysine residues on target proteins. Abnormal expression of KDMs is closely associated with the pathogenesis of various inflammatory diseases such as liver fibrosis, lung injury, and autoimmune diseases. Despite becoming exciting targets for diagnosing and treating these diseases, the role of these enzymes in the regulation of immune and inflammatory response is still unclear. Here, we review the underlying mechanisms through which KDMs regulate immune-related pathways and inflammatory responses. In addition, we also discuss the future applications of KDMs inhibitors in immune and inflammatory diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Drug discovery of histone lysine demethylases (KDMs) inhibitors (progress from 2018 to present).Eur J Med Chem. 2022 Mar 5;231:114143. doi: 10.1016/j.ejmech.2022.114143. Epub 2022 Jan 20. Eur J Med Chem. 2022. PMID: 35101649 Review.
-
Inhibitors of Jumonji C domain-containing histone lysine demethylases overcome cisplatin and paclitaxel resistance in non-small cell lung cancer through APC/Cdh1-dependent degradation of CtIP and PAF15.Cancer Biol Ther. 2022 Dec 31;23(1):65-75. doi: 10.1080/15384047.2021.2020060. Cancer Biol Ther. 2022. PMID: 35100078 Free PMC article.
-
The Emerging Significance of Histone Lysine Demethylases as Prognostic Markers and Therapeutic Targets in Head and Neck Cancers.Cells. 2022 Mar 17;11(6):1023. doi: 10.3390/cells11061023. Cells. 2022. PMID: 35326475 Free PMC article. Review.
-
The emerging role of histone lysine demethylases in prostate cancer.Mol Cancer. 2012 Aug 6;11:52. doi: 10.1186/1476-4598-11-52. Mol Cancer. 2012. PMID: 22867098 Free PMC article. Review.
-
Targeting histone demethylases as a potential cancer therapy (Review).Int J Oncol. 2022 Sep;61(3):103. doi: 10.3892/ijo.2022.5393. Epub 2022 Jul 8. Int J Oncol. 2022. PMID: 35801593 Review.
Cited by
-
Conjunctival MicroRNA Expression Signature in Primary Sjögren's Syndrome Dry Eye: A NanoString-Based Bioinformatic Analysis.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):80. doi: 10.1167/iovs.66.4.80. Invest Ophthalmol Vis Sci. 2025. PMID: 40298889 Free PMC article.
-
Deciphering aging-associated molecular mechanisms in bone marrow derived hematopoietic stem cells in the elderly using NGS data.Bioinformation. 2024 Feb 29;20(2):180-189. doi: 10.6026/973206300200180. eCollection 2024. Bioinformation. 2024. PMID: 38497076 Free PMC article.
-
X and Y Differences in Melanoma Survival Between the Sexes.Pigment Cell Melanoma Res. 2025 Jan;38(1):e13194. doi: 10.1111/pcmr.13194. Epub 2024 Aug 23. Pigment Cell Melanoma Res. 2025. PMID: 39180225 Free PMC article. Review.
-
Transposable element activity captures human pluripotent cell states.EMBO Rep. 2025 Jan;26(2):329-352. doi: 10.1038/s44319-024-00343-y. Epub 2024 Dec 12. EMBO Rep. 2025. PMID: 39668246 Free PMC article.
-
PCAT19: the role in cancer pathogenesis and beyond.Front Cell Dev Biol. 2024 Dec 18;12:1435717. doi: 10.3389/fcell.2024.1435717. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39744012 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

