Measuring Fatigue in Multiple Sclerosis: There may be Trouble Ahead
- PMID: 37353721
- PMCID: PMC10444927
- DOI: 10.1007/s40120-023-00501-9
Measuring Fatigue in Multiple Sclerosis: There may be Trouble Ahead
Abstract
Introduction: Poorly developed patient-reported outcome measures (PROs) risk type-II errors (i.e. false negatives) in clinical trials, resulting in erroneous failure to achieve trial endpoints. Validity is a fundamental requirement of fit-for-purpose PROs, with the main determinant of validity being the PROs items, i.e. content validity. Here, we sought to identify fatigue PRO instruments used in multiple sclerosis (MS) studies and to assess the extent to which their development satisfied current content validity standards.
Methods: We searched Embase® and Medline® for MS studies using fatigue-based PROs. Abstracts were screened, PROs identified, and their relevant development papers assessed against seven Consensus Standards for Measurement Instruments (COSMIN) criteria for content development.
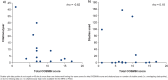
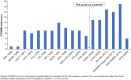
Results: From 3814 abstracts, 18 fatigue PROs met our inclusion criteria. Most PROs did not satisfy at least one COSMIN content validity standard. Frequent omissions during PRO development include: clearly defined constructs; conceptual frameworks; qualitative research in representative samples; and literature reviews. PRO development quality has improved significantly since FDA guidance was published (U = 10.0, p = 0.02). However, scatterplots and correlations between PRO COSMIN scores and citation frequency (rho = - 0.62) and clinical trials usage (rho = + 0.18) implied that PRO quality is unrelated to choice. COSMIN scores implied that the Fatigue Symptoms and Impact Questionnaire-Relapsing Multiple Sclerosis (FSIQ-RMS) and Neurological Fatigue Index-Multiple Sclerosis (NFI-MS) had the strongest evidence for adequate content validity.
Conclusion: Most existing fatigue PROs do not meet COSMIN content validity requirements. Although two PROs scored well on aggregate (NFI-MS and FSIQ-RMS), our subsequent evaluation of the item sets that generated their scores implied that both PROs have weaker content validity than COSMIN suggests. This indicates that COSMIN criteria require further development, and raises significant concerns about how we have measured one of the most common and burdensome MS symptoms. A detailed head-to-head psychometric evaluation is needed to determine the impact of different PRO development qualities and the implications of the problems implied by our analyses, on measurement performance.
Keywords: COSMIN criteria; Content validity; Fatigue; Fatigue measurement; Measurement; Multiple sclerosis; Patient reported outcomes.
Plain language summary
In MS clinical trials, impacts such as fatigue, walking ability, and quality of life, are measured using questionnaires—called patient-reported outcome measures—completed by people living with MS. The quality of these measures is fundamentally important. If poor quality patient-reported outcome measures are used, treatment benefits are easily missed or underestimated.We studied the quality of 18 fatigue patient-reported outcome measures previously used in MS studies. Specifically, we studied how the questionnaire questions were developed and scored them against recognised quality control standards. In general, the patient-reported outcome measures were poor. Only two scored reasonably well. One common weakness was that people living with MS were not involved during patient-reported outcome measure development. We also conducted novel examinations that went beyond the quality control standards. These test how well the questions relate back to the MS impacts they claim to measure. We found even the two best patient-reported outcome measures were poor.Our study had two findings. First, patient-reported outcome measures of MS fatigue are poor. Second, current standards for testing patient-reported outcome measure development are too easy to satisfy, overestimate patient-reported outcome measure quality, and need updating. Therefore, the ways we measure MS fatigue, one of the most common and burdensome MS symptoms, are scientifically weak. Measuring fatigue in multiple sclerosis: there may be trouble ahead—a video abstract (MP4 125165 KB).
© 2023. The Author(s).
Conflict of interest statement
James Close has nothing to disclose. Jo Vandercappellen and Miriam King were employees of Novartis Pharma AG at the time of manuscript preparation. Jeremy Hobart has received consulting fees, honoraria, support to attend meetings or research support from Acorda, Asubio, Bayer Schering, Biogen Idec, F. Hoffmann-La Roche, Genzyme, Merck Serono, Novartis, Oxford PharmaGenesis, and Teva.
Figures
Similar articles
-
Psychometric properties of instruments for measuring abuse of older people in community and institutional settings: A systematic review.Campbell Syst Rev. 2024 Aug 29;20(3):e1419. doi: 10.1002/cl2.1419. eCollection 2024 Sep. Campbell Syst Rev. 2024. PMID: 39211334 Free PMC article. Review.
-
Measuring upper limb function in MS: Which existing patient reported outcomes are fit for purpose?eNeurologicalSci. 2020 Mar 16;19:100237. doi: 10.1016/j.ensci.2020.100237. eCollection 2020 Jun. eNeurologicalSci. 2020. PMID: 32258444 Free PMC article. Review.
-
The suitability of patient-reported outcome measures used to assess the impact of hypoglycaemia on quality of life in people with diabetes: a systematic review using COSMIN methods.Diabetologia. 2021 Jun;64(6):1213-1225. doi: 10.1007/s00125-021-05382-x. Epub 2021 Feb 2. Diabetologia. 2021. PMID: 33528625 Free PMC article.
-
Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance.Health Qual Life Outcomes. 2014 Jul 22;12:116. doi: 10.1186/s12955-014-0116-1. Health Qual Life Outcomes. 2014. PMID: 25048687 Free PMC article. Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
Cited by
-
Clinical trial evidence of quality-of-life effects of disease-modifying therapies for multiple sclerosis: a systematic analysis.J Neurol. 2024 Jun;271(6):3131-3141. doi: 10.1007/s00415-024-12366-5. Epub 2024 Apr 16. J Neurol. 2024. PMID: 38625399 Free PMC article.
-
Detecting fatigue in multiple sclerosis through automatic speech analysis.Front Hum Neurosci. 2024 Sep 13;18:1449388. doi: 10.3389/fnhum.2024.1449388. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39345945 Free PMC article.
-
Global prevalence of fatigue in patients with multiple sclerosis: a systematic review and meta-analysis.Front Neurol. 2024 Oct 2;15:1457788. doi: 10.3389/fneur.2024.1457788. eCollection 2024. Front Neurol. 2024. PMID: 39416662 Free PMC article.
-
[Fatigue in chronic physical diseases].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024 Nov;67(11):1231-1238. doi: 10.1007/s00103-024-03951-0. Epub 2024 Sep 16. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024. PMID: 39284934 Free PMC article. Review. German.
-
Design recommendations for studies that evaluate multiple sclerosis fatigue interventions.Front Psychiatry. 2024 Jan 10;14:1287344. doi: 10.3389/fpsyt.2023.1287344. eCollection 2023. Front Psychiatry. 2024. PMID: 38268567 Free PMC article. No abstract available.
References
-
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous