A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act
- PMID: 37353796
- PMCID: PMC10290406
- DOI: 10.1186/s13023-023-02790-7
A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the Orphan Drug Act
Abstract
Background: Rare diseases affect more than 30 million Americans. The passage of the Orphan Drug Act (ODA) in the United States in 1983 represented a launching point for a rare disease drug development revolution for these patients. Financial incentives provided by the ODA through its Orphan Drug Designation Program, in addition to remarkable scientific advances over the past 40 years, have led to hundreds of drug approvals for rare diseases. Our research examines the rare diseases that have been targeted by orphan drug designations and subsequent approvals since the law was enacted.
Methods: Using an internal FDA database, we classified and analyzed all orphan drug designations and approvals from 1983 to 2022 by disease and therapeutic area.
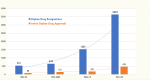
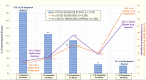
Results: Over the 40 years of the ODA, 6,340 orphan drug designations were granted, representing drug development for 1,079 rare diseases. Additionally, 882 of those designations resulted in at least one FDA approval for use in 392 rare diseases. Much of this development has been concentrated in oncology as seven of the top ten most designated and approved diseases were rare cancers.
Conclusions: Researchers have estimated that there may be 7000-10,000 rare diseases that have been identified and described. Based on our study, we can conclude that around 5% of rare diseases have an FDA-approved drug and up to 15% of rare diseases have at least one drug that has been developed and shown promise in their treatment, diagnosis or prevention. Funding of basic and translational science for rare disease drug development should continue in order to bring therapies to the millions of affected patients who remain without treatment options.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Orphan Drug Act. P.L. 97–414 (Jan 4, 1983).
-
- Orphan Drug Act. 21 USC § 360aa. 1983.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous