Respiratory challenges and ventilatory management in different types of acute brain-injured patients
- PMID: 37353832
- PMCID: PMC10290317
- DOI: 10.1186/s13054-023-04532-4
Respiratory challenges and ventilatory management in different types of acute brain-injured patients
Abstract
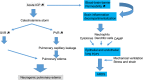
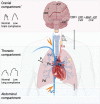
Acute brain injury (ABI) covers various clinical entities that may require invasive mechanical ventilation (MV) in the intensive care unit (ICU). The goal of MV, which is to protect the lung and the brain from further injury, may be difficult to achieve in the most severe forms of lung or brain injury. This narrative review aims to address the respiratory issues and ventilator management, specific to ABI patients in the ICU.
Keywords: Acute brain injury; Acute respiratory distress syndrome; Cerebral autoregulation; Lung protective ventilation; Mechanical ventilation; Neurogenic pulmonary edema.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Robba C, Poole D, McNett M, Asehnoune K, Bösel J, Bruder N, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46(12):2397–2410. doi: 10.1007/s00134-020-06283-0. - DOI - PMC - PubMed

