First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing
- PMID: 37353884
- PMCID: PMC10290200
- DOI: 10.1002/jev2.12332
First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing
Abstract
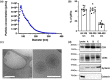
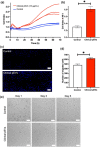
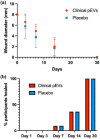
The release of growth factors, cytokines and extracellular matrix modifiers by activated platelets is an important step in the process of healthy wound healing. Extracellular vesicles (EVs) released by activated platelets carry this bioactive cargo in an enriched form, and may therefore represent a potential therapeutic for the treatment of delayed wound healing, such as chronic wounds. While EVs show great promise in regenerative medicine, their production at clinical scale remains a critical challenge and their tolerability in humans is still to be fully established. In this work, we demonstrate that Ligand-based Exosome Affinity Purification (LEAP) chromatography can successfully isolate platelet EVs (pEVs) of clinical grade from activated platelets, which retain the regenerative properties of the parent cell. LEAP-isolated pEVs display the expected biophysical features of EV populations and transport essential proteins in wound healing processes, including insulin growth factor (IGF) and transforming growth factor beta (TGF-ß). In vitro studies show that pEVs induce proliferation and migration of dermal fibroblasts and increase dermal endothelial cells' angiogenic potential, demonstrating their wound healing potential. pEV treatment activates the ERK and Akt signalling pathways within recipient cells. In a first-in-human, double-blind, placebo-controlled, phase I clinical trial of healthy volunteer adults, designed primarily to assess safety in the context of wound healing, we demonstrate that injections of LEAP-purified pEVs in formulation buffer are safe and well tolerated (Plexoval II study, ACTRN12620000944932). As a secondary objective, biological activity in the context of wound healing rate was assessed. In this cohort of healthy participants, in which the wound bed would not be expected to be deficient in the bioactive cargo that pEVs carry, all wounds healed rapidly and completely and no difference in time to wound closure of the treated and untreated wounds was observed at the single dose tested. The outcomes of this study evidence that pEVs manufactured through the LEAP process can be injected safely in humans as a potential wound healing treatment, and warrant further study in clinical trials designed expressly to assess therapeutic efficacy in patients with delayed or disrupted wound healing.
Keywords: extracellular vesicles; first-in-human platelet EV therapy; platelet EVs; wound healing.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
All authors are or were employed or received financial support from Exopharm Ltd. Jancy Johnson, Sam Q. K. Law, Sadman Bhuiyan, Anabel Silva, Melanie Schoppet, Chantelle Blyth, Owen C. Tatford, Anna Cifuentes‐Rius, Patrick F. James, Angus Tester, Ian Dixon, Gregor Lichtfuss are shareholders of Exopharm Ltd.
Figures


Comment in
-
The importance of controlled clinical trials with extracellular vesicles.J Extracell Vesicles. 2023 Jul;12(7):e12347. doi: 10.1002/jev2.12347. J Extracell Vesicles. 2023. PMID: 37477630 Free PMC article. No abstract available.
References
-
- Achar, R. A. N. , Silva, T. C. , Achar, E. , Martines, R. B. , & Machado, J. L. M. (2014). Use of insulin‐like growth factor in the healing of open wounds in diabetic and non‐diabetic rats. Acta Cirúrgica Brasileira, 29, 125–131. - PubMed
-
- Balli, M. , Vitali, F. , Janiszewski, A. , Caluwé, E. , Cortés‐Calabuig, A. , Carpentier, S. , Duelen, R. , Ronzoni, F. , Marcelis, L. , Bosisio, F. M. , Bellazzi, R. , Luttun, A. , De Angelis, M. G. C. , Ceccarelli, G. , Lluis, F. , & Sampaolesi, M. (2020). Autologous micrograft accelerates endogenous wound healing response through ERK‐induced cell migration. Cell Death and Differentiation, 27, 1520–1538. - PMC - PubMed
-
- Baum, C. L. , & Arpey, C. J. (2005). Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatologic Surgery, 31, 674–686. - PubMed
-
- Burden, C. S. , Jin, J. , Aleš, P. , & Bracewell, D. G. (2012). A monolith purification process for virus‐like particles from yeast homogenate. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences, 880, 82–89. - PubMed
-
- Cardeñosa, M. E. , Domínguez‐Maldonado, G. , & Córdoba‐Fernández, A. (2017). Efficacy and safety of the use of platelet‐rich plasma to manage venous ulcers. Journal of Tissue Viability, 26, 138–143. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

